PAUL 10.3.doc
Download as doc, pdf0 likes5 views
The document provides the steps to calculate the density of LiCl using given values. It states that the distance between Li and Cl ions in LiCl is 0.257 nm. It considers each ion to occupy a cubic volume with side length equal to the ion spacing. Using the molecular mass of LiCl, Avogadro's number, and the ion spacing, it calculates the density of LiCl to be 2.0739 g/cm3.
1 of 3
Download to read offline

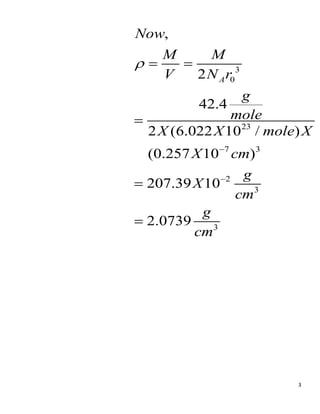
Ad
Recommended
Electrodynamics & Electrical Technology dated 27-06-2024
Electrodynamics & Electrical Technology dated 27-06-2024Rajput AbdulWaheed Bhatti
╠²
Physics Collection 27-06-2024Electronics & Electrodynamics NOTES dated 27-06-2024
Electronics & Electrodynamics NOTES dated 27-06-2024Rajput AbdulWaheed Bhatti
╠²
The document contains a sequential list of numbers from 1 to 66. It appears to be a simple enumeration without any additional context. There are no further details provided regarding the significance or purpose of the numbers.Electrodynamics Notes Young Freedman and Richard Wolfson
Electrodynamics Notes Young Freedman and Richard WolfsonRajput AbdulWaheed Bhatti
╠²
The document consists of a numerical sequence from 1 to 32. It does not provide narrative content or thematic information. The focus appears to be purely on listing numerical values.Electronics and Electrodynamics Notes 27-06-2024
Electronics and Electrodynamics Notes 27-06-2024Rajput AbdulWaheed Bhatti
╠²
The document lists various concepts and principles related to electromagnetics, circuits, and electrical components from multiple textbooks. It includes topics such as amplifiers, capacitance, impedance, and electromagnetic fundamentals, with references to various authors and editions. The extensive compilation highlights key electrical engineering topics and their respective sources.Vectors and Tensor Analysis 11-04-2024.doc
Vectors and Tensor Analysis 11-04-2024.docRajput AbdulWaheed Bhatti
╠²
The document contains two sections about two individuals, Robert C Wrede and Prasun Kumar Nayak. Robert C Wrede's section is brief and ends after line 8. The section about Prasun Kumar Nayak spans lines 9 through 37 and details a 373 page document he authored that ends on line 36. Both sections conclude with "The End".Lagrangian & Hamiltonian Mechanics 11-04-2024.doc
Lagrangian & Hamiltonian Mechanics 11-04-2024.docRajput AbdulWaheed Bhatti
╠²
This document appears to be a list of names, numbers, and short phrases. It includes the names John Tailor, Akhlaq Hussain, Dieter Strach, Wolfgang Notling, Vladimir Pletser, Fowler, and NIVALDO. Various page numbers are mentioned such as 291/413, 385 Daqaq, 371, 655 Pages, and 8888. Short phrases also appear including "Degree of Freedom", "Generalized Momenta", and "Hamiltonian". The document does not provide much context or narrative to summarize.Work Done By F.doc
Work Done By F.docRajput AbdulWaheed Bhatti
╠²
1. The document presents the solution to two mechanics problems (Problems 18 and 19) involving calculating displacement (d) and work (W) given position vectors.
2. For Problem 18, d is calculated as 2 units and W is calculated as 26 Nm.
3. For Problem 19, d is calculated as -1 units and W is calculated as 45 Nm.Visible Light Range.doc
Visible Light Range.docRajput AbdulWaheed Bhatti
╠²
The document summarizes the photon energies of visible light and FM radio frequencies.
(1) The range of photon energies in visible light is from 1.6533 eV to 3.2631 eV.
(2) The energy of a photon from a typical 100 MHz FM radio station broadcast is 4.136├Ś10-15 eV.TEMP of Blackbody.doc
TEMP of Blackbody.docRajput AbdulWaheed Bhatti
╠²
The document calculates the temperature of a blackbody with a peak spectrum of 700 nm. It uses Wien's displacement law, which relates the peak wavelength to the blackbody temperature. It shows the calculations for peak wavelengths in the visible light region at 700 nm, the microwave region at 3 cm, and the FM radio wave region at 3 m. In each case, it applies Wien's law and solves for the temperature in Kelvin. The temperatures calculated are 29,000 K for 700 nm visible light, 3,000 K for 3 cm microwaves, and 30,000 K for 3 m FM radio waves.Ratio Fb & Fg.doc
Ratio Fb & Fg.docRajput AbdulWaheed Bhatti
╠²
The document describes calculating the ratio of the magnetic force to the gravitational force on a proton moving vertically at the Earth's equator. It gives the proton's speed, the horizontal component of Earth's magnetic field, the proton's mass, and the gravitational acceleration. It then shows the calculations of the magnetic force using the magnetic field and speed, and the gravitational force using mass and gravitational acceleration. The ratio of magnetic to gravitational force is calculated to be approximately 16.R of Curvature.doc
R of Curvature.docRajput AbdulWaheed Bhatti
╠²
A beam of particles including protons, electrons, deuterons, and helium atoms all with a speed of 2.5 x 10^8 m/s passes through a magnetic field of 0.40 T perpendicular to their velocity. The radius of curvature of the path is calculated for each particle type using the formula that relates magnetic field, particle mass, charge, and velocity. The radius is found to be 6.52 x 10^-2 m for protons, 3.559 x 10^-1 m for electrons, 1.305 x 10^-1 m for deuterons, and 2.594 x 10^-1 m for helium atoms.Parallel Vectors.doc
Parallel Vectors.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a math problem involving vectors. It shows that the vectors (1,3,3), (1,6,6), and (1,-6,-6) are parallel to each other and equal to zero when their dot products are calculated. This means the vectors are linearly dependent and span a line through the origin in three-dimensional space rather than filling out the full three-dimensional space.JB Marion 8.27.doc
JB Marion 8.27.docRajput AbdulWaheed Bhatti
╠²
The spacecraft orbits Earth with a speed of 10,160 m/s at a perigee of 6,680 km. Using the law of conservation of angular momentum, the speed of the spacecraft at its apogee of 42,200 km can be calculated. Substituting the given values into the angular momentum equation and solving for the apogee speed gives a value of 1,608.26 m/s.Gasiorovicz-3.doc
Gasiorovicz-3.docRajput AbdulWaheed Bhatti
╠²
Ultraviolet light with a wavelength of 350 nm was shone on a potassium surface, ejecting photoelectrons with a maximum kinetic energy of 1.6 eV. Using the formula that relates photon energy, work function, and kinetic energy, along with the given values, the work function of potassium was calculated to be 1.94 eV.Gasiorovicz 4.doc
Gasiorovicz 4.docRajput AbdulWaheed Bhatti
╠²
The document discusses using photoelectric emission data from aluminum to calculate Planck's constant and the work function of aluminum. It provides the maximum kinetic energies of photoelectrons emitted from aluminum when irradiated with 200nm and 258nm wavelength light. It then shows the calculations using Einstein's photoelectric equation to solve for Planck's constant and the work function. Planck's constant is calculated to be 6.626x10-34 Js and the work function of aluminum is calculated to be 3.9 eV.Fowles Cassiday 4.3.doc
Fowles Cassiday 4.3.docRajput AbdulWaheed Bhatti
╠²
The document discusses determining if a given force field is conservative. It provides two examples of force fields and shows that for a force field to be conservative, the curl of the force must be equal to zero at all points. Additionally, a conservative force field can be written as the gradient of a scalar potential function. For the two example force fields provided, the document calculates the curls and determines that they are equal to zero, showing that the forces are conservative and can be written as gradients of potential functions.Fowles Cassiday 4.2.doc
Fowles Cassiday 4.2.docRajput AbdulWaheed Bhatti
╠²
The document discusses conservative and non-conservative force fields. It states that a force field is conservative if its curl is zero everywhere, meaning the gradient of a scalar potential field can represent it. Option (a) represents a conservative field, while option (b) is not conservative because its curl is non-zero. Option (c) also represents a conservative field because its curl is equal to zero.Bright Star.doc
Bright Star.docRajput AbdulWaheed Bhatti
╠²
A bright star has an effective surface temperature of 20,000 K. Using Wien's law, the wavelength with maximum emission is 145 nm. This places it in the ultraviolet region of the electromagnetic spectrum, specifically in the far ultraviolet range between 100-200 nm.Black-Body R.doc
Black-Body R.docRajput AbdulWaheed Bhatti
╠²
1. The document finds the wavelength of blackbody radiation at temperatures of 3 K, 300 K, and 3000 K using Wien's displacement law.
2. At 3 K the wavelength is calculated to be 966 micrometers.
3. At 300 K the wavelength is calculated to be 9.66 millimeters.
4. At 3000 K the wavelength is calculated to be 966 nanometers.Area of a Triangle.doc
Area of a Triangle.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a matrix problem. It involves a 3x3 matrix with entries (1,1,1), (2,3,4), (3,0,1). Several steps of algebraic manipulations are shown to solve for the values of i, j, k, and expressions for X in terms of i, j, k. The final values obtained are i=1, j=3, k=10.Area of a Triangle 22-25.doc
Area of a Triangle 22-25.docRajput AbdulWaheed Bhatti
╠²
The document provides solutions to 5 problems that calculate the area of triangles defined by 3D coordinate points. Each problem lists the 3 points that define the triangle, calculates the vectors between the points, finds the cross product of 2 vectors to obtain the area, and states the final area value.Stephen 4.31.doc
Stephen 4.31.docRajput AbdulWaheed Bhatti
╠²
A muonic atom consists of a muon in place of an electron orbiting the nucleus. For a muon in a hydrogen atom:
(a) The smallest radius of the muon orbit is 151.46 femtometers.
(b) The binding energy of the muon is 2518 electronvolts.
The reduced mass and Bohr model were used to calculate the radius and binding energy from the muon and proton masses.Stephen 4.30.doc
Stephen 4.30.docRajput AbdulWaheed Bhatti
╠²
The document discusses exciting a hydrogen atom from the n=2 state to a higher state using a 397 nm laser. It shows the calculations of the energy differences between states. The highest state the hydrogen atom can be excited to is n=7, as the energy of the 397 nm photon is enough to overcome the difference between the n=2 and n=7 states but not the n=7 and n=8 states.Stephen 4.25.doc
Stephen 4.25.docRajput AbdulWaheed Bhatti
╠²
(1) The binding energy of an electron in the ground state of deuterium is 13.6 eV.
(2) The binding energy of an electron in the ground state of He+ is 54.4 eV.
(3) The binding energy of an electron in the ground state of Be+++ is 217.6 eV.Stephen 4.24.doc
Stephen 4.24.docRajput AbdulWaheed Bhatti
╠²
The initial state of the hydrogen atom was n=1 and the final state was n=5. This is because the photon emitted had a wavelength of 95 nm, which corresponds to an energy difference of 0.55 eV between the initial and final states of the hydrogen atom. The only transition that satisfies this is from the first to the fifth energy level.How payment terms are configured in Odoo 18
How payment terms are configured in Odoo 18Celine George
╠²
Payment terms in Odoo 18 help define the conditions for when invoices are due. This feature can split payments into multiple parts and automate due dates based on specific rules.Nutrition Assessment and Nutrition Education ŌĆō Unit 4 | B.Sc Nursing 5th Seme...
Nutrition Assessment and Nutrition Education ŌĆō Unit 4 | B.Sc Nursing 5th Seme...RAKESH SAJJAN
╠²
This PowerPoint presentation is based on Unit 4 ŌĆō Nutrition Assessment and Nutrition Education, a core topic in the 5th Semester of B.Sc Nursing under the subject Community Health Nursing ŌĆō I, as per the Indian Nursing Council (INC) guidelines.
The unit provides foundational knowledge of nutritional assessment techniques, importance of balanced diets, and health education strategies aimed at improving community nutrition. It empowers future nurses to play a key role in promoting nutrition, preventing malnutrition, and implementing dietary interventions at individual, family, and community levels.
Ō£ģ The PPT covers the following topics in detail:
Introduction to Nutrition and its role in health and disease prevention
Objectives of nutritional assessment in community settings
Types of nutritional assessment ŌĆō Anthropometric, Biochemical, Clinical, and Dietary (ABCD) methods
Tools and techniques used in each type of nutritional assessment
Interpreting growth charts, BMI, MUAC, and dietary recalls
Identification of malnutrition, both undernutrition and overnutrition
Common nutritional deficiencies ŌĆō protein-energy malnutrition, anemia, vitamin A deficiency, iodine deficiency
Principles of nutrition education and behavior change communication
Role of community health nurse in nutrition education during home visits, camps, and school health programs
Use of charts, posters, flashcards, and food models in health teaching
Culturally appropriate and locally available food suggestions
Strategies for promoting infant and young child feeding (IYCF)
National nutrition programs: POSHAN Abhiyaan, Mid-Day Meal Scheme, ICDS, and Anemia Mukt Bharat
Monitoring and evaluation of nutrition interventions
This PPT is perfect for:
B.Sc Nursing students preparing for unit tests or university exams
Nursing educators delivering community health lessons
Field work, community posting presentations, or group health teaching
Health educators and ASHA trainers working on community nutrition
All content is student-friendly, professionally formatted, and aligned with public health priorities and practical nursing roles.More Related Content
More from Rajput AbdulWaheed Bhatti (20)
TEMP of Blackbody.doc
TEMP of Blackbody.docRajput AbdulWaheed Bhatti
╠²
The document calculates the temperature of a blackbody with a peak spectrum of 700 nm. It uses Wien's displacement law, which relates the peak wavelength to the blackbody temperature. It shows the calculations for peak wavelengths in the visible light region at 700 nm, the microwave region at 3 cm, and the FM radio wave region at 3 m. In each case, it applies Wien's law and solves for the temperature in Kelvin. The temperatures calculated are 29,000 K for 700 nm visible light, 3,000 K for 3 cm microwaves, and 30,000 K for 3 m FM radio waves.Ratio Fb & Fg.doc
Ratio Fb & Fg.docRajput AbdulWaheed Bhatti
╠²
The document describes calculating the ratio of the magnetic force to the gravitational force on a proton moving vertically at the Earth's equator. It gives the proton's speed, the horizontal component of Earth's magnetic field, the proton's mass, and the gravitational acceleration. It then shows the calculations of the magnetic force using the magnetic field and speed, and the gravitational force using mass and gravitational acceleration. The ratio of magnetic to gravitational force is calculated to be approximately 16.R of Curvature.doc
R of Curvature.docRajput AbdulWaheed Bhatti
╠²
A beam of particles including protons, electrons, deuterons, and helium atoms all with a speed of 2.5 x 10^8 m/s passes through a magnetic field of 0.40 T perpendicular to their velocity. The radius of curvature of the path is calculated for each particle type using the formula that relates magnetic field, particle mass, charge, and velocity. The radius is found to be 6.52 x 10^-2 m for protons, 3.559 x 10^-1 m for electrons, 1.305 x 10^-1 m for deuterons, and 2.594 x 10^-1 m for helium atoms.Parallel Vectors.doc
Parallel Vectors.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a math problem involving vectors. It shows that the vectors (1,3,3), (1,6,6), and (1,-6,-6) are parallel to each other and equal to zero when their dot products are calculated. This means the vectors are linearly dependent and span a line through the origin in three-dimensional space rather than filling out the full three-dimensional space.JB Marion 8.27.doc
JB Marion 8.27.docRajput AbdulWaheed Bhatti
╠²
The spacecraft orbits Earth with a speed of 10,160 m/s at a perigee of 6,680 km. Using the law of conservation of angular momentum, the speed of the spacecraft at its apogee of 42,200 km can be calculated. Substituting the given values into the angular momentum equation and solving for the apogee speed gives a value of 1,608.26 m/s.Gasiorovicz-3.doc
Gasiorovicz-3.docRajput AbdulWaheed Bhatti
╠²
Ultraviolet light with a wavelength of 350 nm was shone on a potassium surface, ejecting photoelectrons with a maximum kinetic energy of 1.6 eV. Using the formula that relates photon energy, work function, and kinetic energy, along with the given values, the work function of potassium was calculated to be 1.94 eV.Gasiorovicz 4.doc
Gasiorovicz 4.docRajput AbdulWaheed Bhatti
╠²
The document discusses using photoelectric emission data from aluminum to calculate Planck's constant and the work function of aluminum. It provides the maximum kinetic energies of photoelectrons emitted from aluminum when irradiated with 200nm and 258nm wavelength light. It then shows the calculations using Einstein's photoelectric equation to solve for Planck's constant and the work function. Planck's constant is calculated to be 6.626x10-34 Js and the work function of aluminum is calculated to be 3.9 eV.Fowles Cassiday 4.3.doc
Fowles Cassiday 4.3.docRajput AbdulWaheed Bhatti
╠²
The document discusses determining if a given force field is conservative. It provides two examples of force fields and shows that for a force field to be conservative, the curl of the force must be equal to zero at all points. Additionally, a conservative force field can be written as the gradient of a scalar potential function. For the two example force fields provided, the document calculates the curls and determines that they are equal to zero, showing that the forces are conservative and can be written as gradients of potential functions.Fowles Cassiday 4.2.doc
Fowles Cassiday 4.2.docRajput AbdulWaheed Bhatti
╠²
The document discusses conservative and non-conservative force fields. It states that a force field is conservative if its curl is zero everywhere, meaning the gradient of a scalar potential field can represent it. Option (a) represents a conservative field, while option (b) is not conservative because its curl is non-zero. Option (c) also represents a conservative field because its curl is equal to zero.Bright Star.doc
Bright Star.docRajput AbdulWaheed Bhatti
╠²
A bright star has an effective surface temperature of 20,000 K. Using Wien's law, the wavelength with maximum emission is 145 nm. This places it in the ultraviolet region of the electromagnetic spectrum, specifically in the far ultraviolet range between 100-200 nm.Black-Body R.doc
Black-Body R.docRajput AbdulWaheed Bhatti
╠²
1. The document finds the wavelength of blackbody radiation at temperatures of 3 K, 300 K, and 3000 K using Wien's displacement law.
2. At 3 K the wavelength is calculated to be 966 micrometers.
3. At 300 K the wavelength is calculated to be 9.66 millimeters.
4. At 3000 K the wavelength is calculated to be 966 nanometers.Area of a Triangle.doc
Area of a Triangle.docRajput AbdulWaheed Bhatti
╠²
This document provides the solution to a matrix problem. It involves a 3x3 matrix with entries (1,1,1), (2,3,4), (3,0,1). Several steps of algebraic manipulations are shown to solve for the values of i, j, k, and expressions for X in terms of i, j, k. The final values obtained are i=1, j=3, k=10.Area of a Triangle 22-25.doc
Area of a Triangle 22-25.docRajput AbdulWaheed Bhatti
╠²
The document provides solutions to 5 problems that calculate the area of triangles defined by 3D coordinate points. Each problem lists the 3 points that define the triangle, calculates the vectors between the points, finds the cross product of 2 vectors to obtain the area, and states the final area value.Stephen 4.31.doc
Stephen 4.31.docRajput AbdulWaheed Bhatti
╠²
A muonic atom consists of a muon in place of an electron orbiting the nucleus. For a muon in a hydrogen atom:
(a) The smallest radius of the muon orbit is 151.46 femtometers.
(b) The binding energy of the muon is 2518 electronvolts.
The reduced mass and Bohr model were used to calculate the radius and binding energy from the muon and proton masses.Stephen 4.30.doc
Stephen 4.30.docRajput AbdulWaheed Bhatti
╠²
The document discusses exciting a hydrogen atom from the n=2 state to a higher state using a 397 nm laser. It shows the calculations of the energy differences between states. The highest state the hydrogen atom can be excited to is n=7, as the energy of the 397 nm photon is enough to overcome the difference between the n=2 and n=7 states but not the n=7 and n=8 states.Stephen 4.25.doc
Stephen 4.25.docRajput AbdulWaheed Bhatti
╠²
(1) The binding energy of an electron in the ground state of deuterium is 13.6 eV.
(2) The binding energy of an electron in the ground state of He+ is 54.4 eV.
(3) The binding energy of an electron in the ground state of Be+++ is 217.6 eV.Stephen 4.24.doc
Stephen 4.24.docRajput AbdulWaheed Bhatti
╠²
The initial state of the hydrogen atom was n=1 and the final state was n=5. This is because the photon emitted had a wavelength of 95 nm, which corresponds to an energy difference of 0.55 eV between the initial and final states of the hydrogen atom. The only transition that satisfies this is from the first to the fifth energy level.Recently uploaded (20)
How payment terms are configured in Odoo 18
How payment terms are configured in Odoo 18Celine George
╠²
Payment terms in Odoo 18 help define the conditions for when invoices are due. This feature can split payments into multiple parts and automate due dates based on specific rules.Nutrition Assessment and Nutrition Education ŌĆō Unit 4 | B.Sc Nursing 5th Seme...
Nutrition Assessment and Nutrition Education ŌĆō Unit 4 | B.Sc Nursing 5th Seme...RAKESH SAJJAN
╠²
This PowerPoint presentation is based on Unit 4 ŌĆō Nutrition Assessment and Nutrition Education, a core topic in the 5th Semester of B.Sc Nursing under the subject Community Health Nursing ŌĆō I, as per the Indian Nursing Council (INC) guidelines.
The unit provides foundational knowledge of nutritional assessment techniques, importance of balanced diets, and health education strategies aimed at improving community nutrition. It empowers future nurses to play a key role in promoting nutrition, preventing malnutrition, and implementing dietary interventions at individual, family, and community levels.
Ō£ģ The PPT covers the following topics in detail:
Introduction to Nutrition and its role in health and disease prevention
Objectives of nutritional assessment in community settings
Types of nutritional assessment ŌĆō Anthropometric, Biochemical, Clinical, and Dietary (ABCD) methods
Tools and techniques used in each type of nutritional assessment
Interpreting growth charts, BMI, MUAC, and dietary recalls
Identification of malnutrition, both undernutrition and overnutrition
Common nutritional deficiencies ŌĆō protein-energy malnutrition, anemia, vitamin A deficiency, iodine deficiency
Principles of nutrition education and behavior change communication
Role of community health nurse in nutrition education during home visits, camps, and school health programs
Use of charts, posters, flashcards, and food models in health teaching
Culturally appropriate and locally available food suggestions
Strategies for promoting infant and young child feeding (IYCF)
National nutrition programs: POSHAN Abhiyaan, Mid-Day Meal Scheme, ICDS, and Anemia Mukt Bharat
Monitoring and evaluation of nutrition interventions
This PPT is perfect for:
B.Sc Nursing students preparing for unit tests or university exams
Nursing educators delivering community health lessons
Field work, community posting presentations, or group health teaching
Health educators and ASHA trainers working on community nutrition
All content is student-friendly, professionally formatted, and aligned with public health priorities and practical nursing roles.University of Ghana Cracks Down on Misconduct: Over 100 Students Sanctioned
University of Ghana Cracks Down on Misconduct: Over 100 Students SanctionedKweku Zurek
╠²
University of Ghana Cracks Down on Misconduct: Over 100 Students Sanctioned
VCE Literature Section A Exam Response Guide
VCE Literature Section A Exam Response Guidejpinnuck
╠²
This practical guide shows students of Unit 3&4 VCE Literature how to write responses to Section A of the exam. Including a range of examples writing about different types of texts, this guide:
*Breaks down and explains what Q1 and Q2 tasks involve and expect
*Breaks down example responses for each question
*Explains and scaffolds students to write responses for each question
*Includes a comprehensive range of sentence starters and vocabulary for responding to each question
*Includes critical theory vocabulary╠² lists to support Q2 responsesPaper 108 | ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil Disobedience
Paper 108 | ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil DisobedienceRajdeep Bavaliya
╠²
Dive into the powerful journey from ThoreauŌĆÖs 19thŌĆæcentury essay to GandhiŌĆÖs mass movement, and discover how one manŌĆÖs moral stand became the backbone of nonviolent resistance worldwide. Learn how conscience met strategy to spark revolutions, and why their legacy still inspires todayŌĆÖs social justice warriors. Uncover the evolution of civil disobedience. DonŌĆÖt forget to like, share, and follow for more deep dives into the ideas that changed the world.
M.A. Sem - 2 | Presentation
Presentation Season - 2
Paper - 108: The American Literature
Submitted Date: April 2, 2025
Paper Name: The American Literature
Topic: ThoreauŌĆÖs Influence on Gandhi: The Evolution of Civil Disobedience
[Please copy the link and paste it into any web browser to access the content.]
Video Link: https://youtu.be/HXeq6utg7iQ
For a more in-depth discussion of this presentation, please visit the full blog post at the following link: https://rajdeepbavaliya2.blogspot.com/2025/04/thoreau-s-influence-on-gandhi-the-evolution-of-civil-disobedience.html
Please visit this blog to explore additional presentations from this season:
Hashtags:
#CivilDisobedience #ThoreauToGandhi #NonviolentResistance #Satyagraha #Transcendentalism #SocialJustice #HistoryUncovered #GandhiLegacy #ThoreauInfluence #PeacefulProtest
Keyword Tags:
civil disobedience, Thoreau, Gandhi, Satyagraha, nonviolent protest, transcendentalism, moral resistance, Gandhi Thoreau connection, social change, political philosophySustainable Innovation with Immersive Learning
Sustainable Innovation with Immersive LearningLeonel Morgado
╠²
Prof. Leonel and Prof. Dennis approached educational uses, practices, and strategies of using immersion as a lens to interpret, design, and planning educational activities in a sustainable way. Rather than one-off gimmicks, the intent is to enable instructors (and institutions) to be able to include them in their regular activities, including the ability to evaluate and redesign them.
Immersion as a phenomenon enables interpreting pedagogical activities in a learning-agnostic way: you take a stance on the learning theory to follow, and leverage immersion to envision and guide your practice.Tanja Vujicic - PISA for Schools contact Info
Tanja Vujicic - PISA for Schools contact InfoEduSkills OECD
╠²
Tanja Vujicic, Senior Analyst and PISA for SchoolŌĆÖs Project Manager at the OECD spoke at the OECD webinar 'Turning insights into impact: What do early case studies reveal about the power of PISA for Schools?' on 20 June 2025
PISA for Schools is an OECD assessment that evaluates 15-year-old performance on reading, mathematics, and science. It also gathers insights into studentsŌĆÖ learning environment, engagement and well-being, offering schools valuable data that help them benchmark performance internationally and improve education outcomes. A central ambition, and ongoing challenge, has been translating these insights into meaningful actions that drives lasting school improvement. How to Implement Least Package Removal Strategy in Odoo 18 Inventory
How to Implement Least Package Removal Strategy in Odoo 18 InventoryCeline George
╠²
In Odoo, the least package removal strategy is a feature designed to optimize inventory management by minimizing the number of packages open to fulfill the orders. This strategy is particularly useful for the business that deals with products packages in various quantities such as boxes, cartons or palettes. Publishing Your Memoir with Brooke Warner
Publishing Your Memoir with Brooke WarnerBrooke Warner
╠²
Brooke Warner presents on getting published - traditional, hybrid, and self-publishing.
www.memoirnation.comHow to Manage Different Customer Addresses in Odoo 18 Accounting
How to Manage Different Customer Addresses in Odoo 18 AccountingCeline George
╠²
A business often have customers with multiple locations such as office, warehouse, home addresses and this feature allows us to associate with different addresses with each customer streamlining the process of creating sales order invoices and delivery orders.Community Health Nursing Approaches, Concepts, Roles & Responsibilities ŌĆō Uni...
Community Health Nursing Approaches, Concepts, Roles & Responsibilities ŌĆō Uni...RAKESH SAJJAN
╠²
This PowerPoint presentation is based on Unit 6 ŌĆō Community Health Nursing Approaches, Concepts, Roles & Responsibilities of Community Health Nursing Personnel, designed for B.Sc Nursing 5th Semester students under the subject Community Health Nursing ŌĆō I, following the syllabus of the Indian Nursing Council (INC).
This unit focuses on the various approaches in community health, the organizational framework, and the responsibilities of different levels of nursing staff in the healthcare system. It emphasizes the real-world application of nursing principles to provide comprehensive and preventive care to the community.
¤ōś Key Areas Covered in this Presentation:
Introduction to the concept of community health nursing
Approaches to community health:
Nursing Process Approach
Epidemiological Approach
Evidence-Based Approach
Problem-Solving Approach
Nursing Theories in Community Health Practice
Explanation of teamwork and intersectoral coordination
Concept of primary health care and its application in community nursing
Levels of health care delivery ŌĆō primary, secondary, and tertiary care
Home visit process: principles, planning, implementation, and follow-up
Use of community bag and record maintenance
Roles and responsibilities of:
Auxiliary Nurse Midwives (ANMs)
Community Health Officers (CHOs)
Staff Nurses
ASHA workers
Public Health Nurses (PHNs)
Documentation and reporting in community settings
Promotion of health education, immunization, maternal and child health, and nutritional support
Role of nurse in disease surveillance, outbreak control, and health promotion
Ethical principles in community nursing
Coordination with health team members and village health committees
This presentation is useful for:
Nursing students preparing for university theory exams, class tests, or viva
Nursing educators conducting lectures or field discussions
Interns and trainees working in PHCs, sub-centers, or community settings
Community nurses and health educators involved in rural and urban outreach
The content is simplified, clear, and enhanced with point-wise explanations, flowcharts, and field-related examples for better retention and application.LDMMIA Practitioner Student Reiki Yoga S2 Video PDF Without Yogi Goddess
LDMMIA Practitioner Student Reiki Yoga S2 Video PDF Without Yogi GoddessLDM & Mia eStudios
╠²
A bonus dept update. Happy Summer 25 almost. Do Welcome or Welcome back. Our 10th Free workshop will be released the end of this week, June 20th Weekend. All Materials/updates/Workshops are timeless for future students.
ŌÖźOur Monthly Class Roster is 7,141 for 6/21.
ALL students get privacy naturally. Thx Everyone.
ŌÖź Coming to our Shop This Weekend.
Timeless for Future Grad Level Students.
Practitioner Student. Level/Session 2 Packages.
* ŌÖźThe Review & Topics:
* All virtual, adult, education students must be over 18 years to attend LDMMIA eClasses and vStudio Thx.
* Please refer to our Free Workshops anytime for review/notes.
* Orientation Counts as S1 on introduction. Sold Separately as a PDF. Our S2 includes 2 Videos within 2 Mp4s. Sold Separately for Uploading.
* Reiki Is Japanese Energy Healing used Globally.
* Yoga is over 5k years old from India. It hosts many styles, teacher versions, and itŌĆÖs Mainstream now vs decades ago.
* Teaching Vod, 720 Res, Mp4: Yoga Therapy is Reviewed as a Hatha, Classical, Med Yoga (ND) Base. Take practice notes as needed or repeat videos.
* Fused Teaching Vod, 720 Res, Mp4: Yoga Therapy Meets Reiki Review. Take Practice notes as needed or repeat videos.
* Video, 720 Res, Mp4: Practitioner Congrats and Workshop Visual Review with Suggestions.
ŌÖź Bonus Studio Video, 720 Res, Mp4: Our 1st Reiki Video. Produced under Yogi Goddess, LDM Recording. As a Reiki, Kundalini, ASMR Spa, Music Visual. For Our Remastered, Beatz Single for Goddess Vevo Watchers. https://www.reverbnation.com/yogigoddess
* ŌÖź Our Videos are Vevo TV and promoted within the LDMMIA Profiles.
* Scheduled upload for or by Weekend Friday June 13th.
* LDMMIA Digital & Merch Shop: https://ldm-mia.creator-spring.com
* ŌÖź As a student, make sure you have high speed connections/wifi for attendance. This sounds basic, I know lol. But, for our video section. The High Speed and Tech is necessary. Otherwise, any device can be used. Our Zip drive files should serve MAC/PC as well.
* ŌÖź On TECH Emergency: I have had some rare, rough, horrid timed situations as a Remote Student. Pros and Cons to being on campus. So Any Starbucks (coffee shop) or library can be used for wifi hot spots. You can work at your own speed and pace.
* ŌÖź We will not be hosting deadlines, tests/exams.
* ŌÖźAny homework will be session practice and business planning. Nothing stressful or assignment submissions.
Plate Tectonic Boundaries and Continental Drift Theory
Plate Tectonic Boundaries and Continental Drift TheoryMarie
╠²
This 28 slide presentation covers the basics of plate tectonics and continental drift theory. It is an effective introduction into a full plate tectonics unit study, but does not cover faults, stress, seismic waves, or seafloor spreading.
To download PDF, visit The Homeschool Daily. We will be uploading more slideshows to follow this one. Blessings, Marie LDM Recording Presents Yogi Goddess by LDMMIA
LDM Recording Presents Yogi Goddess by LDMMIALDM & Mia eStudios
╠²
A bonus dept update. Happy Summer 25 almost. Do Welcome or Welcome back. Our 10th Free workshop will be released the end of this week, June 20th Weekend. All Materials/updates/Workshops are timeless for future students.
6/17/25: ŌĆ£My now Grads, YouŌĆÖre doing well. I applaud your efforts to continue. We all are shifting to new paradigm realities. Its rough, thereŌĆÖs good and bad days/weeks. However, Reiki with Yoga assistance, does work.ŌĆØ
6/18/25: "For those planning the Training Program Do Welcome. Happy Summer 2k25. You are not ignored and much appreciated. Our updates are ongoing and weekly since Spring. I Hope you Enjoy the Practitioner Grad Level. There's more to come. We will also be wrapping up Level One. So I can work on Levels 2 topics. Please see documents for any news updates. Also visit our websites. Every decade I release a Campus eMap. I will work on that for summer 25. We have 2 old libraries online thats open. https://ldmchapels.weebly.com "
A Safe House,
sanctuary of virtual relaxation and rejuvenation.
By ┬®YogiGoddess of ┬®LDMMIA, ┬®LDMYoga.
ŌÖźTeacher Dept: (Rev Dr) Leslie Moore, ND Yoga (Aide/LPN Trained), Metaphysician,
Using Reiki Practitioner/Master Level Trained.
#yogigoddess @YogiGoddessVEVO
ŌÖźLDMMIA & Depts: are fusing the fan clubs so do welcome.
We are timeless and a safe haven / Cyber Space. ThatŌĆÖs the design of our Fan/Reader/Loyal Blog.ŌÖź
LDM HQ Est. in Ann Arbor, MI 2005.
- Moved to Detroit in 2006,
- Expanded online 2007-2024+
- Became a Beatz Studio in 2009 as Yogi Goddess. After our Apple Podcast
- Relocated to Mount Pleasant MI for College The Pandemic Ending.
- Endemic - Present; Moved back to assist Family in Metro Detroit.
Practitioner Student. Level/Session 2
* The Review & Topics:
* All virtual, adult, education students must be over 18 years to attend LDMMIA eClasses and vStudio Thx.
* Please refer to our Free Workshops anytime for review/notes.
*Tech: Products Sold Separately are for Uploading Size Reasons, THX.
MIA TECH: Videos under Copyright including our Music Video for Yogi Goddess, Can only be picked up vs shop downloaded. We are under vydia.com
Pickup will be our Youtube, for unlisted Playlist.
We do have another Vod for Session 2, Level 1.
After that we move on to Session 3.
Levels 1-3 should be done by August to Sept.
LDM Recording, Yogi Goddess Bio (ReverbNation)
Organization functions as a Studio 1st. We are a Media Co, Private Sector, and Global Listed.
Imagine we are 2 different studios. One for Yoga, the Other for Music Beatz. We are also Vevo TV for Smart TV and Youtube, 2 platforms. The audience differs.
Our Biz income are Media monetization within The Entertainment genre. This includes the category of Yoga, Reiki, ASMR, and Music Beatz. Any other tips, donations, B2C sales/Student Tuition are extra. The Biz gifts are appreciated. (We have been given a few $K for random emergencies.)
Birnagar High School Platinum Jubilee Quiz.pptx
Birnagar High School Platinum Jubilee Quiz.pptxSourav Kr Podder
╠²
Birnagar High School Platinum Jubilee Celebration QuizHow to Customize Quotation Layouts in Odoo 18
How to Customize Quotation Layouts in Odoo 18Celine George
╠²
Customizing quotation layouts in Odoo 18 allows businesses to personalize their quotations to match branding or specific requirements. This can include adding logos, custom fields, or modifying headers and footers. Ad
PAUL 10.3.doc
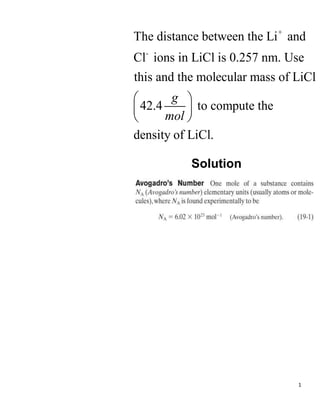
- 1. 1 + - The distance between the Li and Cl ions in LiCl is 0.257 nm. Use this and the molecular mass of LiCl 42.4 to compute the density of LiCl. g mol ’ā” ’āČ ’ā¦ ’āĘ ’ā© ’āĖ Solution
- 2. 2 0 3 0 23 0 We consider each ion to occupy a cubic volume of side r . The ions occupy a volume of 2 , where 6.02 10 is AvogadroŌĆÖs number. The density is thus related to equilibrium spacing r by 2 A A N r N M M V N ’ü▓ ’ü▓ ’ĆĮ ’é┤ ’ĆĮ ’ĆĮ 3 0 (1) Ar ’ĆŁ ’ĆŁ ’ĆŁ 23 7 0 Using 42.4 , 6.022 10 / , 0.257 10 in eq.(1), we get, A g M mole N X mole r X cm ’ĆŁ ’ĆĮ ’ĆĮ ’ĆĮ
- 3. 3 3 0 23 7 3 2 3 3 2 42.4 2 (6.022 10 / ) (0.257 10 ) 207.39 10 2.0739 , A M M V N r g mole X X mole X X cm Now g X cm g cm ’ü▓ ’ĆŁ ’ĆŁ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ ’ĆĮ
