BOYLES LAW.pptx jhghjghghjgjkggghghghghgg
Download as pptx, pdf0 likes51 views
Boyle's Law, established by Robert Boyle in 1662, describes the inverse relationship between gas volume and pressure at constant temperature. The document covers essential properties of gases including pressure, volume, and temperature, with standard units and conversion factors provided. Additionally, it includes various theoretical problems and examples demonstrating the application of Boyle's Law in calculating gas behavior under changing conditions.
1 of 24
Download to read offline














Ad
Recommended
BOYLES LAW.pptx
BOYLES LAW.pptxJane360787
╠²
1. The document describes various gases and their properties including pressure, volume, temperature, and mass. It provides definitions and conversion formulas for units of pressure, volume, and temperature.
2. Boyle's law is introduced, which states that the pressure and volume of a gas are inversely proportional at constant temperature. An example problem demonstrates how to use the Boyle's law equation to relate initial and final pressure and volume.
3. The relationship between pressure and volume of a gas is investigated through example problems applying Boyle's law, such as calculating new volume given a change in pressure.Roberts Boyle's Law Principles and explainations
Roberts Boyle's Law Principles and explainationsRayYan950549
╠²
Robert Boyle studied the relationship between the volume and pressure of gases in 1662. Boyle's Law states that the pressure of a gas is inversely proportional to its volume when temperature is held constant. The document then provides details on the standard units used to measure gas properties like pressure, volume, and temperature. It also presents sample problems demonstrating how to use Boyle's Law to calculate changes in gas volume or pressure when one variable is altered while temperature remains constant.thegaslawscomplete-120227061412-phpapp01.pdf
thegaslawscomplete-120227061412-phpapp01.pdfnona wayne dela pena
╠²
- Boyle's law states that the volume of a gas is inversely proportional to its pressure when temperature is kept constant. It describes the relationship between the pressure and volume of a gas.
- Charles' law describes how the volume of a gas changes with temperature. It states that the volume of a gas increases or decreases proportionally with an increase or decrease in its temperature.
- The combined gas law combines Boyle's and Charles' laws and describes the relationship between pressure, volume, and temperature for a gas.Gas laws (Boyle and Charles' Laws) handout
Gas laws (Boyle and Charles' Laws) handoutAfael
╠²
The document summarizes four gas laws:
1. Boyle's law states that the volume of a gas is inversely proportional to its pressure when temperature is kept constant.
2. Charles' law describes the direct relationship between the volume and temperature of a gas when pressure is constant.
3. Several sample problems demonstrate how to use the formulas for each gas law to calculate volume or pressure given values for two variables.
4. Practice problems provide additional examples for readers to work through the calculations.The gas laws complete
The gas laws completeChristian Sampaga
╠²
1) Boyle's law states that the volume of a gas is inversely proportional to its pressure when temperature is kept constant. Charles' law describes the direct relationship between volume and temperature of a gas at constant pressure. Gay-Lussac's law explains that pressure of a gas rises with increasing temperature at constant volume.
2) The combined gas law incorporates Boyle's, Charles's and Gay-Lussac's laws to describe the interrelationships between pressure, volume and temperature for a fixed amount of gas.
3) According to Avogadro's law, equal volumes of gases under same conditions of temperature and pressure contain equal numbers of molecules. Dalton's law states that total pressure of a gas mixture is the sumthe gas laws
the gas lawsvxiiayah
╠²
The document discusses several gas laws including Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, the combined gas law, and the ideal gas law. It provides definitions of these laws and examples of calculations using each one. The key relationships covered are: the inverse relationship between pressure and volume at constant temperature (Boyle's law), the direct relationship between volume and temperature at constant pressure (Charles' law), the direct relationship between pressure and temperature at constant volume (Gay-Lussac's law), the relationship between volume and amount of gas at constant pressure and temperature (Avogadro's law), and the ideal gas law which relates pressure, volume, temperature,Boyles law module in the grade 10 science
Boyles law module in the grade 10 sciencefloriejanemacaya1
╠²
This document explains Boyle's Law and its relation to the kinetic molecular theory, detailing how gas pressure and volume are interrelated. It describes key measurable properties of gases, standard conditions (STP), and the mathematical representation of Boyle's Law. The document includes assessment questions related to these concepts to gauge understanding.BEHAVIOR OF GASES 4TH QUARTER_POWER .pptx
BEHAVIOR OF GASES 4TH QUARTER_POWER .pptxRoyoMel
╠²
The document outlines an experiment using a syringe to demonstrate Boyle's Law, which states that the volume of a gas is inversely proportional to its pressure at a constant temperature. It provides definitions for key terms such as pressure, volume, and temperature, along with sample problems that apply Boyle's Law in real-world scenarios. The educational objectives include describing gas behavior and solving problems related to changes in gas conditions.Unit 5 - Gases.pptx
Unit 5 - Gases.pptxGraceJobelDeJesus
╠²
1. The document discusses the fundamental properties and laws governing gases, including pressure, volume, temperature, amount of gas, and how they relate based on Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, and the ideal gas law.
2. Key concepts covered include the definition of pressure, different pressure units, relationships between pressure and volume, relationships between temperature and volume, and how the number of gas molecules affects volume.
3. Examples are provided to demonstrate how to use the gas laws to calculate pressure, volume, temperature, or amount of gas under different conditions.Ch5 Gases
Ch5 GasesSa'ib J. Khouri
╠²
1. Gases have no definite shape or volume but take the shape of their container. Gas particles are in constant random motion and collide with each other and the container walls.
2. The kinetic molecular theory provides an explanation for gas behavior at the molecular level. It states that gas particles are in constant random motion and exert pressure due to collisions with container walls.
3. The gas laws describe the macroscopic behavior of gases through relationships between pressure, volume, temperature, and amount of gas. The kinetic molecular theory qualitatively explains the gas laws based on gas particle motion and interactions.Chapter 5 notes
Chapter 5 notesWong Hsiung
╠²
Gases exist as individual molecules that are in constant random motion. The kinetic molecular theory describes gases as composed of molecules that are separated by large distances and move rapidly in random directions, frequently colliding with one another. The theory states that the average kinetic energy of gas molecules is proportional to the absolute temperature of the gas. Higher temperatures cause molecules to move faster on average with more molecules possessing higher speeds.BoyleŌĆÖs and Charles' Law problem solving ppt.pptx
BoyleŌĆÖs and Charles' Law problem solving ppt.pptxANNCHERYLNARCISO1
╠²
This document provides examples applying Boyle's law to calculate gas properties such as volume and pressure under different conditions. It includes 16 sample problems where the initial volume and pressure or temperature of a gas is given and the question asks to calculate the corresponding value of one of the other properties after a change based on the inverse relationship between pressure and volume described by Boyle's law.Physical Characteristics Of Gases
Physical Characteristics Of Gasesshawnschlueter
╠²
The document summarizes the kinetic molecular theory and gas laws relating pressure, temperature, volume and amount of gases. It defines key terms like ideal gas, diffusion and effusion. The kinetic molecular theory has 5 assumptions including gases being made of particles in random motion with no interparticle forces. Gas laws discussed include Boyle's law, Charles' law, Gay-Lussac's law and combined gas law. Dalton's law of partial pressures states the total pressure of a gas mixture equals the sum of partial pressures of individual gases.State Of Matter
State Of Matterwraithxjmin
╠²
The document discusses the three states of matter - solid, liquid, and gas. It explains the properties of gases and how gas particles are in constant random motion. The gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas equation are described. It also covers gas pressure, measurement of pressure using barometers and manometers, gas density calculations, and sample problems involving the gas laws.Boyle law problems
Boyle law problemsJoan Torres-Algo
╠²
The document contains multiple problems and solutions related to Boyle's Law, which states that pressure and volume of a gas are inversely proportional at constant temperature. It includes various scenarios involving different gases, their volumes and pressures, and asks for calculations based on Boyle's Law. Additionally, the document provides pressure conversion factors to aid in problem-solving.States of matter
States of matterHoshi94
╠²
1. The document discusses the different states of matter and summarizes the key differences between gases, liquids, and solids.
2. It then covers various gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas equation.
3. The kinetic molecular theory is introduced to explain gas behavior at the molecular level in terms of molecule motion and interactions.Gen. Chem 1 - gas laws.pptx
Gen. Chem 1 - gas laws.pptxHermyFeliciano
╠²
The document discusses the kinetic molecular theory of gases and properties of gases. Some key points:
1) Gases are composed of molecules that are in constant random motion and interact through perfectly elastic collisions. The average kinetic energy is proportional to temperature.
2) Gas pressure is caused by molecular collisions with container walls. Pressure increases with higher temperature or lower volume based on the gas laws.
3) The kinetic molecular theory explains gas properties and laws such as Boyle's, Charles', Avogadro's, and Dalton's through consideration of molecular motion and collisions.
4) Gas density, pressure, volume, temperature, and amount relationships can be described using the ideal gas law. Real gases deviate fromUnit 4: Behavior of Gases
Unit 4: Behavior of GasesJimnaira Abanto
╠²
The document outlines key concepts related to gas behavior, including Boyle's Law, Charles's Law, and Gay-Lussac's Law, along with the ideal gas equation. It explains how gases behave under various conditions of pressure, volume, and temperature, and includes problem-solving examples to illustrate these laws. Additionally, it discusses Avogadro's principle regarding the relationship between gas volume and the number of moles at constant temperature and pressure.Gas laws
Gas lawsHanna Mae Hernani
╠²
1. The document summarizes several gas laws: Boyle's law describes the inverse relationship between the pressure and volume of a gas at constant temperature. Charles's law describes the direct relationship between volume and temperature of a gas at constant pressure. Gay-Lussac's law describes the direct relationship between pressure and temperature of a gas at constant volume. The combined gas law incorporates all three relationships.
2. Sample problems demonstrate applying the gas laws to calculate unknown pressure, volume, or temperature given values for two variables. Formulas are provided for deriving relationships between the variables under each gas law.GASES: BEHAVIOR AND CALCULATIONS
GASES: BEHAVIOR AND CALCULATIONSINSTITUTO TECNOL├ōGICO DE SONORA
╠²
Here are the densities of the gases at STP:
Hydrogen: 0.0899 g/L or 0.0899 g/m3
Oxygen: 1.429 g/L or 1.429 g/m3
Chlorine: 3.214 g/L or 3.214 g/m3
Radon: 9.73 g/L or 9.73 g/m35,gases
5,gasesž╣┘ä┘Ŗ ž╣┘ä┘Ŗ
╠²
This chapter discusses gases and their properties. It covers the three states of matter, the composition of the atmosphere, and how gases differ from liquids and solids in their ability to disperse uniformly and mix completely. The document then examines gas pressure, how it is measured using barometers and manometers, and several gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas law. These laws relate the volume, pressure, temperature, and quantity of a gas.sch3u_u5_lesson_2_boyles_law powerpoint.ppt
sch3u_u5_lesson_2_boyles_law powerpoint.pptseok81
╠²
The kinetic molecular theory explains that all substances consist of tiny particles in constant motion, with gases exhibiting the most movement among the states of matter. Robert Boyle pioneered quantitative gas measurements, establishing Boyle's Law, which states that the product of a gas's pressure and volume remains constant at a constant temperature. Modern pressure units are now defined in pascals, with standard conditions referred to as SATP, set at 25┬░C and 100kPa.Gas-Laws.pptx gas charles boyle gay lussac
Gas-Laws.pptx gas charles boyle gay lussacrenald7
╠²
The document discusses several gas laws including Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, and the combined gas law. Boyle's law states that the volume of a gas is inversely proportional to its pressure when temperature is kept constant. Charles' law specifies that the volume of a gas increases as temperature increases if pressure is kept constant. Gay-Lussac's law indicates pressure and temperature have a direct relationship if volume remains fixed. The combined gas law incorporates each of these relationships. Avogadro's law concerns the direct relationship between volume and amount of gas at constant temperature and pressure.Chapter 10 Lecture- Gases
Chapter 10 Lecture- GasesMary Beth Smith
╠²
1) Gases expand to fill their containers, are highly compressible, and have low densities due to the large distances between molecules. Their physical properties are similar regardless of chemical properties.
2) Pressure is caused by molecular collisions with surfaces. It increases with more frequent or forceful collisions. Temperature increases collision frequency and force. Pressure also rises with increased amount or decreased volume of gas.
3) Kinetic molecular theory explains gas behavior by modeling gases as particles in random motion, where temperature corresponds to average kinetic energy. This enables understanding of gas laws and pressure in terms of molecular collisions.Ap Chem: Unit 5: Gases
Ap Chem: Unit 5: Gasesbobcatchemistry
╠²
The document discusses key concepts about gases from the kinetic molecular theory and gas laws. It introduces gases in the atmosphere and how they were studied historically. It then covers gas pressure, units of pressure, Boyle's law, Charles' law, Avogadro's law, the ideal gas law, gas stoichiometry, Dalton's law of partial pressures, and the kinetic molecular theory of gases. Examples are provided to demonstrate calculations using these gas laws and concepts.Combined gas law
Combined gas lawBernabeCeleste
╠²
PCO2
Ptotal = PN2 + PO2 + PCO2
2.50 atm = 0.90 atm + 0.60 atm + PCO2
PCO2 = 2.50 atm - 0.90 atm - 0.60 atm
PCO2 = 1 atm
So the partial pressure of CO2 is 1 atm.gas_laws.ppt
gas_laws.pptaruniyerBitcoinminer
╠²
The document discusses the key gas laws and concepts:
1) Gases are highly compressible, occupy their container fully, exert uniform pressure, diffuse easily, and have low densities according to kinetic molecular theory.
2) The gas laws describe the relationships between pressure, volume, temperature, and moles of gas including Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's principle, and the combined ideal gas law.
3) The ideal gas law combines all gas laws as PV=nRT, relating pressure, volume, moles of gas, temperature, and the universal gas constant R. It can be used to calculate gas properties.mirrorrs, concave convex. formula solution
mirrorrs, concave convex. formula solutionBaltazarRosales1
╠²
The document provides tips to reduce gadget exposure, including setting screen time limits, creating gadget-free zones, and engaging in offline activities. It discusses the effects of various types of radiation, emphasizing that long exposure to harmful radiation can lead to health issues. Additionally, it explains the laws of reflection and characteristics of images formed by plane mirrors.Biomolecules.pptxBiomolecules.pptxBiomolecules.pptx
Biomolecules.pptxBiomolecules.pptxBiomolecules.pptxBaltazarRosales1
╠²
Biomolecules are complex organic molecules essential for life, categorized into carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates serve as energy sources and structural components, proteins facilitate numerous cellular functions and reactions, lipids provide energy storage and cell membrane structure, while nucleic acids contain genetic information and direct protein synthesis. Each class of biomolecules is composed of specific elements and structural units, highlighting their critical roles in biological systems.More Related Content
Similar to BOYLES LAW.pptx jhghjghghjgjkggghghghghgg (20)
BEHAVIOR OF GASES 4TH QUARTER_POWER .pptx
BEHAVIOR OF GASES 4TH QUARTER_POWER .pptxRoyoMel
╠²
The document outlines an experiment using a syringe to demonstrate Boyle's Law, which states that the volume of a gas is inversely proportional to its pressure at a constant temperature. It provides definitions for key terms such as pressure, volume, and temperature, along with sample problems that apply Boyle's Law in real-world scenarios. The educational objectives include describing gas behavior and solving problems related to changes in gas conditions.Unit 5 - Gases.pptx
Unit 5 - Gases.pptxGraceJobelDeJesus
╠²
1. The document discusses the fundamental properties and laws governing gases, including pressure, volume, temperature, amount of gas, and how they relate based on Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, and the ideal gas law.
2. Key concepts covered include the definition of pressure, different pressure units, relationships between pressure and volume, relationships between temperature and volume, and how the number of gas molecules affects volume.
3. Examples are provided to demonstrate how to use the gas laws to calculate pressure, volume, temperature, or amount of gas under different conditions.Ch5 Gases
Ch5 GasesSa'ib J. Khouri
╠²
1. Gases have no definite shape or volume but take the shape of their container. Gas particles are in constant random motion and collide with each other and the container walls.
2. The kinetic molecular theory provides an explanation for gas behavior at the molecular level. It states that gas particles are in constant random motion and exert pressure due to collisions with container walls.
3. The gas laws describe the macroscopic behavior of gases through relationships between pressure, volume, temperature, and amount of gas. The kinetic molecular theory qualitatively explains the gas laws based on gas particle motion and interactions.Chapter 5 notes
Chapter 5 notesWong Hsiung
╠²
Gases exist as individual molecules that are in constant random motion. The kinetic molecular theory describes gases as composed of molecules that are separated by large distances and move rapidly in random directions, frequently colliding with one another. The theory states that the average kinetic energy of gas molecules is proportional to the absolute temperature of the gas. Higher temperatures cause molecules to move faster on average with more molecules possessing higher speeds.BoyleŌĆÖs and Charles' Law problem solving ppt.pptx
BoyleŌĆÖs and Charles' Law problem solving ppt.pptxANNCHERYLNARCISO1
╠²
This document provides examples applying Boyle's law to calculate gas properties such as volume and pressure under different conditions. It includes 16 sample problems where the initial volume and pressure or temperature of a gas is given and the question asks to calculate the corresponding value of one of the other properties after a change based on the inverse relationship between pressure and volume described by Boyle's law.Physical Characteristics Of Gases
Physical Characteristics Of Gasesshawnschlueter
╠²
The document summarizes the kinetic molecular theory and gas laws relating pressure, temperature, volume and amount of gases. It defines key terms like ideal gas, diffusion and effusion. The kinetic molecular theory has 5 assumptions including gases being made of particles in random motion with no interparticle forces. Gas laws discussed include Boyle's law, Charles' law, Gay-Lussac's law and combined gas law. Dalton's law of partial pressures states the total pressure of a gas mixture equals the sum of partial pressures of individual gases.State Of Matter
State Of Matterwraithxjmin
╠²
The document discusses the three states of matter - solid, liquid, and gas. It explains the properties of gases and how gas particles are in constant random motion. The gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas equation are described. It also covers gas pressure, measurement of pressure using barometers and manometers, gas density calculations, and sample problems involving the gas laws.Boyle law problems
Boyle law problemsJoan Torres-Algo
╠²
The document contains multiple problems and solutions related to Boyle's Law, which states that pressure and volume of a gas are inversely proportional at constant temperature. It includes various scenarios involving different gases, their volumes and pressures, and asks for calculations based on Boyle's Law. Additionally, the document provides pressure conversion factors to aid in problem-solving.States of matter
States of matterHoshi94
╠²
1. The document discusses the different states of matter and summarizes the key differences between gases, liquids, and solids.
2. It then covers various gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas equation.
3. The kinetic molecular theory is introduced to explain gas behavior at the molecular level in terms of molecule motion and interactions.Gen. Chem 1 - gas laws.pptx
Gen. Chem 1 - gas laws.pptxHermyFeliciano
╠²
The document discusses the kinetic molecular theory of gases and properties of gases. Some key points:
1) Gases are composed of molecules that are in constant random motion and interact through perfectly elastic collisions. The average kinetic energy is proportional to temperature.
2) Gas pressure is caused by molecular collisions with container walls. Pressure increases with higher temperature or lower volume based on the gas laws.
3) The kinetic molecular theory explains gas properties and laws such as Boyle's, Charles', Avogadro's, and Dalton's through consideration of molecular motion and collisions.
4) Gas density, pressure, volume, temperature, and amount relationships can be described using the ideal gas law. Real gases deviate fromUnit 4: Behavior of Gases
Unit 4: Behavior of GasesJimnaira Abanto
╠²
The document outlines key concepts related to gas behavior, including Boyle's Law, Charles's Law, and Gay-Lussac's Law, along with the ideal gas equation. It explains how gases behave under various conditions of pressure, volume, and temperature, and includes problem-solving examples to illustrate these laws. Additionally, it discusses Avogadro's principle regarding the relationship between gas volume and the number of moles at constant temperature and pressure.Gas laws
Gas lawsHanna Mae Hernani
╠²
1. The document summarizes several gas laws: Boyle's law describes the inverse relationship between the pressure and volume of a gas at constant temperature. Charles's law describes the direct relationship between volume and temperature of a gas at constant pressure. Gay-Lussac's law describes the direct relationship between pressure and temperature of a gas at constant volume. The combined gas law incorporates all three relationships.
2. Sample problems demonstrate applying the gas laws to calculate unknown pressure, volume, or temperature given values for two variables. Formulas are provided for deriving relationships between the variables under each gas law.GASES: BEHAVIOR AND CALCULATIONS
GASES: BEHAVIOR AND CALCULATIONSINSTITUTO TECNOL├ōGICO DE SONORA
╠²
Here are the densities of the gases at STP:
Hydrogen: 0.0899 g/L or 0.0899 g/m3
Oxygen: 1.429 g/L or 1.429 g/m3
Chlorine: 3.214 g/L or 3.214 g/m3
Radon: 9.73 g/L or 9.73 g/m35,gases
5,gasesž╣┘ä┘Ŗ ž╣┘ä┘Ŗ
╠²
This chapter discusses gases and their properties. It covers the three states of matter, the composition of the atmosphere, and how gases differ from liquids and solids in their ability to disperse uniformly and mix completely. The document then examines gas pressure, how it is measured using barometers and manometers, and several gas laws including Boyle's law, Charles' law, Avogadro's law, and the ideal gas law. These laws relate the volume, pressure, temperature, and quantity of a gas.sch3u_u5_lesson_2_boyles_law powerpoint.ppt
sch3u_u5_lesson_2_boyles_law powerpoint.pptseok81
╠²
The kinetic molecular theory explains that all substances consist of tiny particles in constant motion, with gases exhibiting the most movement among the states of matter. Robert Boyle pioneered quantitative gas measurements, establishing Boyle's Law, which states that the product of a gas's pressure and volume remains constant at a constant temperature. Modern pressure units are now defined in pascals, with standard conditions referred to as SATP, set at 25┬░C and 100kPa.Gas-Laws.pptx gas charles boyle gay lussac
Gas-Laws.pptx gas charles boyle gay lussacrenald7
╠²
The document discusses several gas laws including Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's law, and the combined gas law. Boyle's law states that the volume of a gas is inversely proportional to its pressure when temperature is kept constant. Charles' law specifies that the volume of a gas increases as temperature increases if pressure is kept constant. Gay-Lussac's law indicates pressure and temperature have a direct relationship if volume remains fixed. The combined gas law incorporates each of these relationships. Avogadro's law concerns the direct relationship between volume and amount of gas at constant temperature and pressure.Chapter 10 Lecture- Gases
Chapter 10 Lecture- GasesMary Beth Smith
╠²
1) Gases expand to fill their containers, are highly compressible, and have low densities due to the large distances between molecules. Their physical properties are similar regardless of chemical properties.
2) Pressure is caused by molecular collisions with surfaces. It increases with more frequent or forceful collisions. Temperature increases collision frequency and force. Pressure also rises with increased amount or decreased volume of gas.
3) Kinetic molecular theory explains gas behavior by modeling gases as particles in random motion, where temperature corresponds to average kinetic energy. This enables understanding of gas laws and pressure in terms of molecular collisions.Ap Chem: Unit 5: Gases
Ap Chem: Unit 5: Gasesbobcatchemistry
╠²
The document discusses key concepts about gases from the kinetic molecular theory and gas laws. It introduces gases in the atmosphere and how they were studied historically. It then covers gas pressure, units of pressure, Boyle's law, Charles' law, Avogadro's law, the ideal gas law, gas stoichiometry, Dalton's law of partial pressures, and the kinetic molecular theory of gases. Examples are provided to demonstrate calculations using these gas laws and concepts.Combined gas law
Combined gas lawBernabeCeleste
╠²
PCO2
Ptotal = PN2 + PO2 + PCO2
2.50 atm = 0.90 atm + 0.60 atm + PCO2
PCO2 = 2.50 atm - 0.90 atm - 0.60 atm
PCO2 = 1 atm
So the partial pressure of CO2 is 1 atm.gas_laws.ppt
gas_laws.pptaruniyerBitcoinminer
╠²
The document discusses the key gas laws and concepts:
1) Gases are highly compressible, occupy their container fully, exert uniform pressure, diffuse easily, and have low densities according to kinetic molecular theory.
2) The gas laws describe the relationships between pressure, volume, temperature, and moles of gas including Boyle's law, Charles' law, Gay-Lussac's law, Avogadro's principle, and the combined ideal gas law.
3) The ideal gas law combines all gas laws as PV=nRT, relating pressure, volume, moles of gas, temperature, and the universal gas constant R. It can be used to calculate gas properties.More from BaltazarRosales1 (17)
mirrorrs, concave convex. formula solution
mirrorrs, concave convex. formula solutionBaltazarRosales1
╠²
The document provides tips to reduce gadget exposure, including setting screen time limits, creating gadget-free zones, and engaging in offline activities. It discusses the effects of various types of radiation, emphasizing that long exposure to harmful radiation can lead to health issues. Additionally, it explains the laws of reflection and characteristics of images formed by plane mirrors.Biomolecules.pptxBiomolecules.pptxBiomolecules.pptx
Biomolecules.pptxBiomolecules.pptxBiomolecules.pptxBaltazarRosales1
╠²
Biomolecules are complex organic molecules essential for life, categorized into carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates serve as energy sources and structural components, proteins facilitate numerous cellular functions and reactions, lipids provide energy storage and cell membrane structure, while nucleic acids contain genetic information and direct protein synthesis. Each class of biomolecules is composed of specific elements and structural units, highlighting their critical roles in biological systems.science 9.. chemistry.pptxjbjhjjkjhkjhjkhjkh
science 9.. chemistry.pptxjbjhjjkjhkjhjkhjkhBaltazarRosales1
╠²
This document discusses the key principles of electron configuration: the Pauli Exclusion Principle limits each orbital to two electrons spinning in opposite directions; Hund's Rule states that orbitals in a sublevel are filled with unpaired electrons before pairing occurs; and the Aufbau Principle builds an atom's configuration by adding electrons into orbitals of increasing energy, such as filling n=1 before n=2. Noble gas notation is also mentioned.electronic configuration.pptx ghjjkgjhjhklh
electronic configuration.pptx ghjjkgjhjhklhBaltazarRosales1
╠²
Electron configuration is the systematic arrangement of electrons in an atom's energy levels and sublevels. The Pauli Exclusion Principle limits each orbital to two electrons with opposite spins. Hund's Rule states that for a given sublevel, electrons occupy orbitals singly with parallel spins before pairing. The Aufbau Principle shows that electrons fill lower energy orbitals before higher ones.PARTS OF MALE REPRODUCTIVE SYSTEM.pptxvhgg
PARTS OF MALE REPRODUCTIVE SYSTEM.pptxvhggBaltazarRosales1
╠²
The document summarizes the major parts of the male reproductive system, including the scrotum, testes, epididymis, vas deferens, ejaculatory duct, urethra, and penis. It describes the development and maturation of sperm cells within the testes and along the epididymis, vas deferens, and ejaculatory duct. It also explains that testosterone is the main male sex hormone responsible for male sexual development and characteristics.OCCURENCE OF EVOLUTION.pptx how does evolution happens
OCCURENCE OF EVOLUTION.pptx how does evolution happensBaltazarRosales1
╠²
This document discusses several key concepts related to the occurrence and mechanisms of evolution:
1. It outlines Jean Baptiste de Lamarck and Charles Darwin's influential theories of evolution, including Lamarck's theories of need, use and disuse, and acquired characteristics as well as Darwin's theory of natural selection.
2. It explores the genetic basis of evolution through concepts like population genetics, gene flow, allele frequencies, non-random mating, and the Hardy-Weinberg equilibrium.
3. It examines various evolutionary patterns such as natural selection, genetic drift, speciation, punctuated equilibrium, microevolution, coevolution, convergent evolution, and adaptive radiation.EVIDENCE OF EVOLUTION.pptx history of evolution
EVIDENCE OF EVOLUTION.pptx history of evolutionBaltazarRosales1
╠²
This document provides evidence of evolution through figures showing similarities in human and animal anatomy. Figure 6 shows the human digestive system with the appendix. Figure 7 shows a snake pelvis bone with a reduced hind limb. Figures 8 and 8B show that early embryonic stages and advanced embryos of different vertebrates share similar structures, providing evidence they evolved from a common ancestor.NERVOUS SYSTEM.pptx tdtffykgygigguiggugiug
NERVOUS SYSTEM.pptx tdtffykgygigguiggugiugBaltazarRosales1
╠²
The nervous system is composed of two main divisions - the central nervous system consisting of the brain and spinal cord, and the peripheral nervous system consisting of nerves that connect to all parts of the body. It coordinates feedback mechanisms through the peripheral and central nervous systems to maintain homeostasis in the body. The nervous system regulates internal processes like temperature, hunger, and organ function to keep the body's conditions stable.MUTATION.pptx learn different ways of mutation how does it happen
MUTATION.pptx learn different ways of mutation how does it happenBaltazarRosales1
╠²
The DNA is used to complete protein synthesis which has two stages - transcription and translation. During translation at the ribosome, messenger RNA sequences are read and translated into amino acids which then form proteins. A mutation is a change in the DNA sequence that can occur either from mistakes when DNA copies or environmental factors like UV light and smoking, and takes place during DNA replication which can then cause abnormal transcription into mRNA.PROTIEN SYNTHESIS.pptx help to understand the process of protien synthesis
PROTIEN SYNTHESIS.pptx help to understand the process of protien synthesisBaltazarRosales1
╠²
DNA contains genetic codes made up of nucleotide bases that are arranged in triplets to code for amino acids. RNA carries these genetic codes from DNA and uses them to assemble amino acids into proteins according to the code. Together, DNA and RNA work through this process of protein synthesis to produce the proteins specified by the genetic code.festival Dancing and Fitness.pptx........
festival Dancing and Fitness.pptx........BaltazarRosales1
╠²
The FITT principle provides an acronym to describe exercise parameters: Frequency refers to the number of weekly sessions; Intensity indicates the difficulty level which can be light, moderate, or vigorous; Time is the duration of each session; and Type specifies the mode of exercise or activity.The Fundamental Body Movements.pptx GFGF
The Fundamental Body Movements.pptx GFGFBaltazarRosales1
╠²
Fundamental body movements are the basic building blocks for more complex physical activities like sports and dancing. They include locomotor movements that move the body through space, such as walking, running, and hopping, as well as non-locomotor movements that involve movement of body parts without traveling, like bending, twisting, and swinging. Students must master fundamental movements like dribbling and kicking during early childhood in order to participate in physical activities as they grow older. The document outlines the main categories and examples of fundamental locomotor and non-locomotor movements, as well as elements of rhythm, space, and qualities of movement important for dance.The Endocrine System.pptx GFTFTFYFUYUYYUY
The Endocrine System.pptx GFTFTFYFUYUYYUYBaltazarRosales1
╠²
Here are the answers to fill in the blanks:
1. Pituitary gland
2. Thyroid gland
3. Parathyroid gland
4. Parathyroid gland
5. Pancreas
6. Thymus gland
7. Thymus gland
8. Adrenal gland
9. Birth control pills
10. Prolactin and OxytocinMyself on Street and Hip-hop Dances.pptx
Myself on Street and Hip-hop Dances.pptxBaltazarRosales1
╠²
1. The document introduces the importance of active recreation for achieving a healthy body and discusses street and hip-hop dances.
2. It provides a series of true/false statements to test the reader's knowledge of concepts like rate of perceived exertion, vigorous vs. passive activities, nutrition, and more.
3. The reader is then asked to identify which physical activities can help sustain fitness by writing "YES" or "NO" next to each one listed, such as watching TV, playing volleyball, eating fruits/veggies, dancing, etc.ARTS 9 L1.pptxHJFJLSKJFKSJGKSDJGKSJJIRJGIJIG
ARTS 9 L1.pptxHJFJLSKJFKSJGKSDJGKSJJIRJGIJIGBaltazarRosales1
╠²
This document discusses media-based arts and design in the Philippines. It notes that photography is considered both a tool for communication and an art form. The document outlines key elements of filmmaking such as the director, actors, cinematography, editing, production design. It also briefly mentions notable Philippine photographers and filmmakers.ART NEOCLASSICISM AND ROMANTISMISISM.pptx
ART NEOCLASSICISM AND ROMANTISMISISM.pptxBaltazarRosales1
╠²
Neoclassicism and Romanticism were artistic periods between the late 18th and early 19th centuries that had distinct characteristics and elements. Neoclassicism took inspiration from Ancient Greek and Roman art, emphasizing historical accuracy and order. Romanticism was an emotional reaction that celebrated nature and heightened sensations through dramatic compositions. Both periods influenced painting, sculpture, and architecture styles during this time.asexual reproduction ppt.pptx
asexual reproduction ppt.pptxBaltazarRosales1
╠²
This document defines asexual reproduction and describes its various types. It explains that asexual reproduction involves a single parent producing offspring that are genetically identical. Various types of asexual reproduction are described, including fission, fragmentation, budding, parthenogenesis, spore production, and vegetative propagation. Examples of each type are provided. The objectives are to define asexual reproduction, describe its different types, and classify organisms by their reproductive methods.Ad
Recently uploaded (20)
The Emergence of Signatures of AGI: The Physics of Learning
The Emergence of Signatures of AGI: The Physics of LearningCharles Martin
╠²
A talk for the Cybernetic Society
International Journal of Pharmacological Sciences (IJPS)
International Journal of Pharmacological Sciences (IJPS)journalijps98
╠²
Call for Research Articles.!!!
FREE PUBLICATION CHARGES
International Journal of Pharmacological Sciences (IJPS)
Webpage URL : https://www.wireilla.com/medical/IJPS/index.html
Wikicfp Url:http://www.wikicfp.com/cfp/servlet/event.showcfp?eventid=181292©ownerid=33993
Authors are invited to submit papers through the Journal Submission System
http://allcfps.com/wireilla/submission/index.php
Submission Deadline : June 17, 2025
Contact Us
Here's where you can reach us : journalijps98@gmail.com or ijpsjournal@wireilla.comRole of Glutamate, glutamine and Alanine in Transport of Ammonia in Tissues
Role of Glutamate, glutamine and Alanine in Transport of Ammonia in TissuesTayyab
╠²
This slide explains the roles of Glutamate, Glutamine, and Alanine in safely transporting toxic ammonia from tissues to the liver and kidneys, where it is detoxified or excreted."Synthesis and characterization of Thiazole derivatives of N-substituted lsatin
Synthesis and characterization of Thiazole derivatives of N-substituted lsatinProfessional Content Writing's
╠²
Thiazole derivatives of N-substituted isatin have attracted significant interest due to their wide-ranging applications in medicinal chemistry, pharmaceuticals, and materials science. These compounds exhibit diverse biological activities, making them promising drug candidates, while their unique chemical structures offer potential in designing advanced functional materials. This presentation focuses on the synthesis and characterization of these derivatives through targeted chemical reactions involving various substituents on the isatin and thiazole cores, enabling the fine-tuning of their biological and physical properties. Characterization techniques such as NMR, FT-IR, Mass Spectrometry, and X-ray crystallography are employed to confirm molecular structures and analyze solid-state properties. These methods provide critical insights into the structureŌĆōactivity relationships of the synthesized compounds. Our presentation highlights the synthetic pathways, structural elucidation, and potential applications of thiazole-based N-substituted isatin derivatives, aiming to support ongoing advancements in drug discovery and material development.
About Author:
Noor Zulfiqar is an award-winning chemist, Premium member of American Chemical Society (ACS), certified publisher & peer reviewer, and an experienced academic lecturer. As a professional content creator, she offers top-tier presentation design, research writing, and scientific content development services. Her multidisciplinary expertise spans computational science, chemistry, nanotechnology, environmental studies, socio-economics, human resource management, life sciences, engineering management, medical and pharmaceutical sciences, and business, her work ensures clarity, creativity, and academic excellence. Her services are ideal for those seeking impactful, visually compelling content tailored to diverse academic and research needs.
For collaborations or custom-designed academic content, feel free to reach out!
Contact:
Email: professionalwriter94@outlook.com
Facebook: https://www.facebook.com/share/1LgwpoyLDg/
Website: https://professional-content-writings.jimdosite.comScience Experiment: Properties of Water.pptx
Science Experiment: Properties of Water.pptxmarionrada1985
╠²
Different experiments to know the properties of waterScientific Instruments Market (PPT) (Satiyam).pptx
Scientific Instruments Market (PPT) (Satiyam).pptxExpert Market Research
╠²
The global scientific instruments market reached USDŌĆ»39.94ŌĆ»billion in 2024 and is projected to grow at a 4.50% CAGR, hitting around USDŌĆ»62.03ŌĆ»billion by 2034.This surge is driven by increasing R&D investments in pharmaceuticals, biotech, environmental testing, and industrial analytics. Demand for high-precision analytical and clinical analyzers supports growth across academic, industrial, and government sectors, fueled by stricter regulations and scientific collaborations worldwide.Smart Grids Selected Topics, Advanced Metering Infrastructure
Smart Grids Selected Topics, Advanced Metering InfrastructureFrancisSeverineRugan
╠²
This slides are for the smart grid AMI componentTISSUE TRANSPLANTATTION and IT'S IMPORTANCE IS DISCUSSED
TISSUE TRANSPLANTATTION and IT'S IMPORTANCE IS DISCUSSEDPhoebeAkinyi1
╠²
Tissue transplant in immunologyAbzymes mimickers in catalytic reactions at nanoscales
Abzymes mimickers in catalytic reactions at nanoscalesOrchideaMariaLecian
╠²
Title: Abzymes mimickers in
catalytic reactions at nanoscales
Speaker: Orchidea Maria Lecian
Authors: Orchidea Maria Lecian, Sergey Suchkov
Talk presented at 4th International Conference on
Advanced Nanomaterials and Nanotechnology
12-13 June 2025, Rome, Italy on 12 June 2025.CULTIVATION - HARVESTING - PROCESSING - STORAGE -.pdf
CULTIVATION - HARVESTING - PROCESSING - STORAGE -.pdfNistarini College, Purulia (W.B) India
╠²
This presentation offers a brief idea about the cultivation, processing, storage and marketing of medicinal plants for the health care practices in sustainable health. The role of different factors has been assessed for the same.Primary and Secondary immune modulation.pptx
Primary and Secondary immune modulation.pptxdevikasanalkumar35
╠²
Primary and secondary immune modulation
GBSN_ Unit 1 - Introduction to Microbiology
GBSN_ Unit 1 - Introduction to MicrobiologyAreesha Ahmad
╠²
Microbiology for Nursing students - According to New PNC course curriculum - 2025Cloud Collaboration Market Challenges, Drivers, Trends, and Forecast by 2031
Cloud Collaboration Market Challenges, Drivers, Trends, and Forecast by 2031moresonali406
╠²
The report is segmented by Component (Solution, Service); Deployment (Private Cloud, Public Cloud, Hybrid Cloud); Organization Size (Large Enterprises, Small and Medium Enterprises (SMEs)); Vertical (BFSI, Consumer Goods And Retail, Education, Government and Public Sector, Healthcare and Life Sciences, Manufacturing, Media and Entertainment, Telecommunication and ITES, Others). The global analysis is further broken-down at regional level and major countries. The report offers the value in USD for the above analysis and segmentsSynthesis and characterization of Thiazole derivatives of N-substituted lsatin
Synthesis and characterization of Thiazole derivatives of N-substituted lsatinProfessional Content Writing's
╠²
Ad
BOYLES LAW.pptx jhghjghghjgjkggghghghghgg
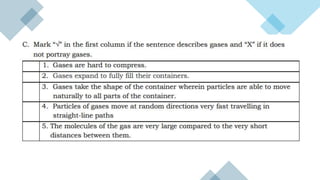
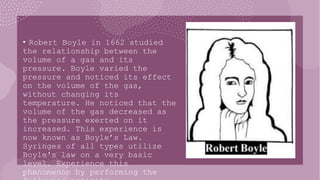
- 5. ŌĆó Robert Boyle in 1662 studied the relationship between the volume of a gas and its pressure. Boyle varied the pressure and noticed its effect on the volume of the gas, without changing its temperature. He noticed that the volume of the gas decreased as the pressure exerted on it increased. This experience is now known as BoyleŌĆÖs Law. Syringes of all types utilize Boyle's law on a very basic level. Experience this phenomenon by performing the
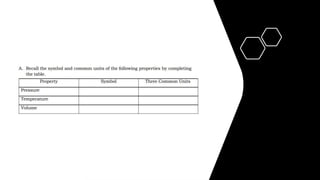
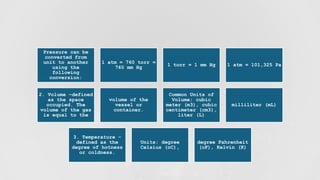
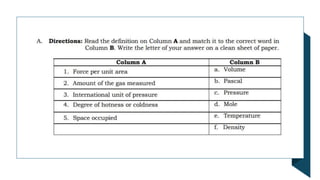
- 6. Measurable Properties of Gases ŌĆó 1. Pressure - the force exerted by the gas on the walls of its container divided by the ŌĆó surface area of the container. ŌĆó The common units of pressure are the following: ŌĆó Pascal (Pa) - standard unit of pressure under System├® International (SI) ŌĆó which is equivalent to a force of one newton (1N = 1 kg m/s2) acting on an ŌĆó area of one square meter. ŌĆó Atmosphere (atm) ŌĆó Torr ŌĆó Millimeter mercury (mm Hg)
- 7. Pressure can be converted from unit to another using the following conversion: 1 atm = 760 torr = 760 mm Hg 1 torr = 1 mm Hg 1 atm = 101,325 Pa 2. Volume ŌĆōdefined as the space occupied. The volume of the gas is equal to the volume of the vessel or container. Common Units of Volume: cubic meter (m3), cubic centimeter (cm3), liter (L) milliliter (mL) 3. Temperature ŌĆō defined as the degree of hotness or coldness. Units: degree Celsius (oC), degree Fahrenheit (oF), Kelvin (K)
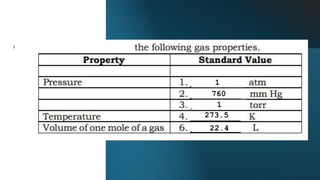
- 8. ŌĆó The temperature of a gas together with its atmospheric pressure differ from place to place and from time to time. As the volume of a gas is dependent on its temperature and pressure, it is significant to have a set of standard conditions for these quantities. This set of standard condition is named as standard temperature and pressure or simply STP. The standard temperature is 0 Ōü░C or 273.15 K and the standard pressure is 1 atm pressure. This is the freezing point of pure water at sea level atmospheric pressure. At STP, one mole of gas occupies 22.4 L of volume.
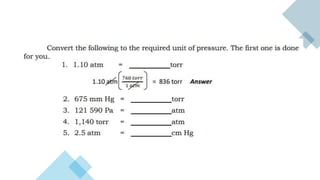
- 10. ŌĆó B. The SI unit of pressure is the Pascal, Pa, but in the V-P relationship of gases, it ŌĆó is necessary to be familiarized with the different units of pressures and the ŌĆó corresponding values for unit conversion. ŌĆó 1 atm = 760 torr ŌĆó 1 atm = 760 mm Hg ŌĆó 1 atm = 101,325 Pa ŌĆó 1 atm = 76 cm Hg
- 14. ŌĆó Activity 2. Gaze, Compare, and Conclude Suppose we have a theoretical gas confined in a jar with a piston at the top as seen in the illustration below. The left side indicates the initial state while that on the right shows the final state after slowly adding weights to the top of the piston. The unit of volume is in cubic meters (m3), while that of pressure is kilopascal (kPa). The
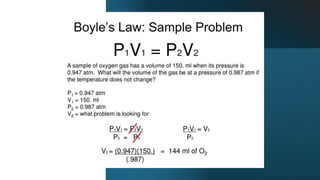
- 19. Example #1: 2.00 L of a gas is at 740.0 mmHg pressure. What is its volume at standard pressure? Example #2: 5.00 L of a gas is at 1.08 atm. What pressure is obtained when the volume is 10.0 L? Example #3: 9.48 L of a gas was at an unknown pressure. However, at standard pressure, its volume was measured to be 8.00 L. What was the unknown pressure? Example #4: If we have 6.00 cm3 of gas at a pressure of 10.0 N/cm2 and we increase the pressure to 20.0 N/cm2, what volume will the gas occupy? Example #5: What pressure is required to compress 196.0 liters of air at 1.00 atmosphere into a cylinder whose volume is 26.0 liters? Example #6: The volume of a gas is 6.10 L, measured at 1.00 atm. What is the pressure of the gas in mmHg if the volume is changed to 9.74 L? Example #7: At 46.0 ┬░C a sample of ammonia gas exerts a pressure of 5.30 atm. What is the pressure when the volume of the gas is reduced to one-eighth (0.125) of the original value at the same temperature? Example #8: In order to measure the volume of a piece of apparatus, a chemist filled a 750. mL flask with 46.65 kPa pressure of gas, then expanded it into the apparatus. The final pressure was 14.95 kPa. Calculate the total volume occupied by the gas.
- 20. Problem #16: 4.00 L of a gas are under a pressure of 6.00 atm. What is the volume of the gas at 2.00 atm? Problem #17: A gas occupies 25.3 mL at a pressure of 790.5 mmHg. Determine the volume if the pressure is reduced to 0.804 atm. Problem #18: A sample of gas has a volume of 12.0 L and a pressure of 1.00 atm. If the pressure of gas is increased to 2.00 atm, what is the new volume of the gas? Problem #19: A container of oxygen has a volume of 30.0 mL and a pressure of 4.00 atm. If the pressure of the oxygen gas is reduced to 2.00 atm and the temperature is kept constant, what is the new volume of the oxygen gas? Problem #20: A tank of nitrogen has a volume of 14.0 L and a pressure of 760.0 mmHg. Find the volume of the nitrogen when its pressure is changed to 400.0 mmHg while the temperature is held constant. Problem #21: A 40.0 L tank of ammonia has a pressure of 8.00 atm. Calculate the volume of the ammonia if its pressure is changed to 12.0 atm while its temperature remains constant. Problem #22: Two hundred liters of helium at 2.00 atm and 28.0 ┬░C is placed into a tank with an internal pressure of 600.0 kPa. Find the volume of the helium after it is compressed into the tank when the temperature of the tank remains 28.0 ┬░C.
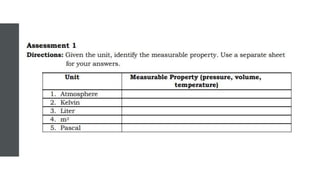
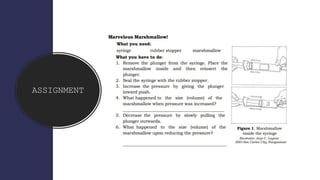
- 22. ASSIGNMENT
- 24. THANK YOU !!!