electronic configuration.pptx ghjjkgjhjhklh
Download as pptx, pdf0 likes7 views
Electron configuration is the systematic arrangement of electrons in an atom's energy levels and sublevels. The Pauli Exclusion Principle limits each orbital to two electrons with opposite spins. Hund's Rule states that for a given sublevel, electrons occupy orbitals singly with parallel spins before pairing. The Aufbau Principle shows that electrons fill lower energy orbitals before higher ones.
1 of 9
Download to read offline




Ad
Recommended
science 9.. chemistry.pptxjbjhjjkjhkjhjkhjkh
science 9.. chemistry.pptxjbjhjjkjhkjhjkhjkhBaltazarRosales1
Ã˝
This document discusses the key principles of electron configuration: the Pauli Exclusion Principle limits each orbital to two electrons spinning in opposite directions; Hund's Rule states that orbitals in a sublevel are filled with unpaired electrons before pairing occurs; and the Aufbau Principle builds an atom's configuration by adding electrons into orbitals of increasing energy, such as filling n=1 before n=2. Noble gas notation is also mentioned.Chapter 4.3 : Electron Configuration
Chapter 4.3 : Electron ConfigurationChris Foltz
Ã˝
The document outlines objectives for learning about electron configuration including listing the number of electrons in each main energy level, stating the Aufbau principle, Pauli exclusion principle, and Hund's rule, and describing electron configurations using orbital, electron-configuration, and noble-gas notations. It then defines electron configuration and the ground state and provides rules for electron configuration including the Aufbau principle, Pauli exclusion principle, and Hund's rule.Electronic configuration Aufbau principle.pdf
Electronic configuration Aufbau principle.pdfjayashreeghuge1
Ã˝
The document discusses atomic models, focusing on Bohr's model which introduces specific orbits for electrons and their electronic configurations, governed by principles like the Aufbau and Hund's rules. It highlights Bohr's limitations in explaining chemical bonding and electron behavior, leading to the concept of quantum mechanics which incorporates electron duality and probability. The document also elaborates on quantum numbers and their role in defining electron properties and configurations.Electron configuration.ppt
Electron configuration.pptCharliez Jane Soriano
Ã˝
Electron configuration refers to the distribution of electrons in an atom's orbitals and energy levels. There are three main principles that determine electron configuration: 1) Electrons have higher energy further from the nucleus and in higher principal quantum shells, 2) Orbitals within a shell have set ordering of energies (s < p < d < f), and 3) Each orbital can hold a maximum of two electrons of opposite spin. The Aufbau principle, Pauli exclusion principle, and Hund's rule further define how electrons fill the orbitals in atoms.Characterizing the Electrons
Characterizing the ElectronsRachel Espino
Ã˝
The document discusses the characteristics of electrons and their arrangements in atomic orbitals, essential for understanding elemental properties. It explains key principles such as Pauli's exclusion principle, Aufbau principle, and Hund's rule, which govern the behavior and distribution of electrons in energy levels and sublevels. It emphasizes the significance of the periodic table in determining electron configurations for elements.Quantum Mechanics
Quantum Mechanicsguest471a994
Ã˝
This document provides an overview of quantum mechanics concepts related to light and atomic structure. It discusses how light behaves as both a wave and particle, and introduces the electromagnetic spectrum. It then covers atomic structure concepts like electron configurations, energy levels, quantum numbers, and orbital shapes and filling diagrams. The document aims to explain how electrons are arranged in atoms and the underlying quantum mechanical principles.Bell301
Bell301Prashant Kumar
Ã˝
The document discusses the Bohr model of the atomic structure, which proposes that electrons orbit the nucleus in defined shells corresponding to specific energy levels. It notes several limitations of the Bohr model, including that it violates the Heisenberg uncertainty principle and cannot explain phenomena like the Zeeman effect. More modern quantum mechanical models of the atom use probabilistic electron orbitals and sublevels within energy shells to better describe atomic structure and spectra.Electron configuration-Grade 9 Science.pptx
Electron configuration-Grade 9 Science.pptxAlvinJuanino
Ã˝
Electron configuration-Grade 9 Science.pptxPauli exclusion principle
Pauli exclusion principleNaheemPinchoos
Ã˝
Wolfgang Pauli formulated the Pauli Exclusion Principle in 1925, for which he received the Nobel Prize in 1945. The principle states that no two electrons in an atom can have the same set of quantum numbers, meaning each electron must have its own unique spin state. This explains the electron configurations of atoms and the periodic table. The principle is fundamental to quantum mechanics and is important for understanding chemical bonding and the properties of solids.Applied Chemistry, atomic and molecular structure, part 1, by Shiraz mahbob PhD
Applied Chemistry, atomic and molecular structure, part 1, by Shiraz mahbob PhDMaqsoodAhmadKhan5
Ã˝
The document provides an overview of atomic and molecular structure, covering topics such as atomic number, isotopes, and the arrangement of electrons in orbitals. It explains fundamental principles like the Pauli exclusion principle, Hund's rule, and the Aufbau principle in filling atomic orbitals, as well as the periodic table and trends in properties across periods and groups. Additionally, it touches on chemical bonding concepts, including ionic and covalent bonds, polarity, and VSEPR theory for predicting molecular shape.MODULE-1-Quantum-mechanical-Model-of-Atom-Copy.pptx
MODULE-1-Quantum-mechanical-Model-of-Atom-Copy.pptxabajabajajaba21
Ã˝
MODULE-1-Quantum-mechanical-Model-of-Atom-Copy.pptxchemical bonding and molecular structure
chemical bonding and molecular structure Akarshik Banerjee
Ã˝
This document presents information about chemical bonding from the chemistry project of students Akarshik Banerjee, Pratyush Dey, and Sayantan Biswas. It discusses various types of chemical bonds including covalent bonds, which form when atoms share electron pairs, and ionic bonds, which form through complete electron transfer. It also describes concepts like the octet rule, Lewis dot structures, formal charge, resonance structures, and molecular geometry based on valence shell electron pair repulsion theory. Hybridization and molecular orbitals are explained as well. In summary, the document provides an overview of key concepts in chemical bonding from the perspective of high school chemistry students.Qrt-2-Week-1-Module-1-Lesson-1.pptx
Qrt-2-Week-1-Module-1-Lesson-1.pptxMATRIXGAMING8
Ã˝
This document provides an overview of the quantum mechanical model of the atom. It discusses key concepts like the development of atomic models, electron configuration, quantum numbers, electron orbitals, and energy levels. Some key points:
- The quantum mechanical model describes the probable locations of electrons in an atom and how they can have discrete energy levels. It improved upon earlier planetary and Bohr models.
- Electrons occupy specific orbitals and energy levels denoted by quantum numbers like principal and azimuthal quantum numbers.
- Electron configuration notation specifies the distribution of electrons across orbitals based on Aufbau's principle and other rules.
- Transitions between energy levels explain the emission or absorption of photons and thus the colorsModels
ModelsGalen West
Ã˝
The document discusses the evolution of atomic models over time from Dalton's model to the current quantum mechanical model. It summarizes key developments including the plum pudding model, Bohr's model of electrons in orbits, and how the Schrodinger equation led to the quantum mechanical model where electrons occupy distinct energy levels and orbitals. The modern model describes electron probability clouds rather than set orbits and accounts for properties like electron spin and the Pauli exclusion principle.Electron_Energy_Level_Notes.ppt
Electron_Energy_Level_Notes.pptCYRILCONSTANTINO2
Ã˝
1) Electrons in atoms occupy different energy levels rather than following classical orbital models. Higher energy levels are farther from the nucleus.
2) Energy levels are divided into sublevels which have different shapes designated by letters. Electrons fill these sublevels according to specific rules.
3) The aufbau principle and Pauli exclusion principle govern how electrons fill atomic orbitals based on energy and allowing no more than two electrons of opposite spin per orbital. Hund's rule favors occupying each orbital in a sublevel singly before pairing electrons.SCI-9-Quarter-2- electron configuration.pptx
SCI-9-Quarter-2- electron configuration.pptxpauloalegria3
Ã˝
The document discusses electron configuration, which refers to the arrangement of electrons in an atom's energy levels and is vital for understanding atomic properties and interactions. Key principles like the Aufbau principle, Pauli exclusion principle, and Hund's rule govern how electrons are distributed among orbitals. Additionally, the document explains how electron configurations affect reactivity and bonding in elements.Electronic-configuration for last term .pptx
Electronic-configuration for last term .pptxivaratot
Ã˝
The document provides an overview of electron configurations and orbital energy levels in hydrogen and many-electron atoms, emphasizing how energy depends on quantum numbers and various interactions in the atom. It describes ground states, excited states, and the application of principles such as Hund's rule and the Pauli exclusion principle in determining electron arrangements. Additionally, it explains the order of filling atomic orbitals based on the aufbau principle and how these configurations relate to the periodic table.Ch 5 electrons in atoms notes
Ch 5 electrons in atoms notesEsther Herrera
Ã˝
The document discusses the quantum mechanical model of the atom. It explains that electrons can occupy certain allowed energy levels around the nucleus, represented by principal quantum numbers. Within these levels are smaller energy sublevels that determine the shape of an atom's orbitals. Electrons fill these orbitals based on specific rules, resulting in unique electron configurations for each element. The configurations and valence electrons allow atoms to gain or lose energy through emission or absorption of photons in quantized amounts, explaining atomic emission spectra.Test
Testrajashekhar2000
Ã˝
The document discusses the atomic structure and models proposed by scientists over time. It explains that the atom consists of a nucleus and electrons. Rutherford proposed that the nucleus is at the center of the atom and contains protons and neutrons, while electrons orbit the nucleus. Bohr's atomic model built on Planck's quantum theory by postulating that electrons orbit in fixed energy levels and can only absorb or emit photons of energy equal to the difference between orbit levels. The model helped explain atomic stability and emission spectra but had limitations for multi-electron atoms.chemical bonding ##@ Abhay
chemical bonding ##@ AbhayAbhay Shah
Ã˝
This document discusses different chemical bonding theories including:
- Covalent bonding which occurs when atoms share electron pairs
- Ionic bonding which involves a complete transfer of electrons between atoms
- Lewis structures which represent bonding using shared electron pairs and the octet rule
- VSEPR theory which predicts molecular geometry based on electron pair repulsion
- Hybridization which involves mixing of atomic orbitals to form new hybrid orbitals
- Molecular orbital theory where atomic orbitals combine to form molecular orbitals of lower energy, providing stable bonding.QUARTER 2 SCIENCE 9.pptx hhfcdlif97v/ vgjyt
QUARTER 2 SCIENCE 9.pptx hhfcdlif97v/ vgjytjeanmycamontalbo
Ã˝
The document outlines various atomic theories, starting with John Dalton's solid sphere model, which posits that all matter is composed of indivisible atoms that differ by element. It progresses through significant models by Thomson, Rutherford, Bohr, and Schrodinger, each building on the understanding of atomic structure, from the distribution of charges to the electron cloud concept. Additionally, it discusses electron configurations and quantum numbers that define the arrangement and behavior of electrons in an atom.B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...Rai University
Ã˝
The document discusses rules for electron arrangement in orbitals. It covers three main principles:
1) Aufbau's principle states that electrons fill atomic orbitals in order of increasing energy. Electrons occupy the lowest energy orbitals first.
2) Pauli's exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This limits each orbital to holding two electrons with opposite spins.
3) Hund's rule states that when multiple degenerate orbitals are available at the same energy level, electrons will occupy them singly with parallel spins before pairing up. This results in the highest possible spin state.B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...Rai University
Ã˝
This document discusses the rules for electron arrangement in orbitals. It covers three main principles:
1) Aufbau's principle, which states that electrons fill atomic orbitals in order of increasing energy.
2) Pauli's exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. This limits each orbital to holding two electrons with opposite spins.
3) Hund's rule, which specifies that electrons first occupy degenerate orbitals singly with parallel spins before pairing up. This maximizes the multiplicity of electrons in orbitals of equal energy.B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...Rai University
Ã˝
This document discusses the rules for electron arrangement in orbitals. It covers three main principles:
1) Aufbau's principle, which states that electrons fill atomic orbitals in order of increasing energy.
2) Pauli's exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. This limits each orbital to holding two electrons with opposite spins.
3) Hund's rule, which specifies that electrons first occupy degenerate orbitals singly with parallel spins before pairing up. This maximizes the multiplicity of electrons in orbitals of equal energy.Lesson 5 - Electronic Configuration.pptx
Lesson 5 - Electronic Configuration.pptxDoveLalune
Ã˝
The document discusses key concepts in atomic structure, including the organization of electrons in energy levels and principles for filling atomic orbitals such as the Aufbau principle, Hund's rule, and the Pauli exclusion principle. It outlines how to write electron configurations and provides examples for various elements. Overall, it serves as a guide to understanding electron distribution in atoms.Quantum Numbers (1).pdf
Quantum Numbers (1).pdfRoxette Rosete
Ã˝
The document summarizes the four quantum numbers that describe electrons: principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). It then discusses electron configuration, which is the arrangement of electrons in an atom based on the Aufbau principle, Pauli exclusion principle, and Hund's rule. Electron configuration can be written using symbols, orbital diagrams, noble gas notation, and electron-dot structures.Electron configuration
Electron configurationMariaisa Apellidos
Ã˝
Electron configuration represents the arrangement of electrons in an atom's orbital shells and subshells. There are three main rules that determine electron configuration: the Aufbau principle, which states that orbitals are filled in order of increasing energy; the Pauli exclusion principle, where no two electrons can have the same quantum numbers; and Hund's rule, where orbitals in a subshell are singly occupied with parallel spins before pairing. Electron configuration can be written out or represented through orbital diagrams, the periodic table, or Möller diagrams.Electron Structure
Electron StructureTrevor23
Ã˝
The document discusses the Bohr model of the hydrogen atom. It explains that the electron can only orbit the nucleus in fixed energy levels called orbits. The lowest energy level is the ground state, with higher levels being excited states. When an electron moves to a higher level, energy is absorbed, and when it moves to a lower level, a photon of energy is released. The wavelength and frequency of light are related to the energy of photons by Planck's constant and the speed of light. Quantum numbers describe the properties of atomic orbitals and electrons, including principal, orbital, and spin quantum numbers. Electron configurations show how electrons are arranged in orbitals according to Aufbau principle, Hund's rule, and Pauli exclusionmirrorrs, concave convex. formula solution
mirrorrs, concave convex. formula solutionBaltazarRosales1
Ã˝
The document provides tips to reduce gadget exposure, including setting screen time limits, creating gadget-free zones, and engaging in offline activities. It discusses the effects of various types of radiation, emphasizing that long exposure to harmful radiation can lead to health issues. Additionally, it explains the laws of reflection and characteristics of images formed by plane mirrors.Biomolecules.pptxBiomolecules.pptxBiomolecules.pptx
Biomolecules.pptxBiomolecules.pptxBiomolecules.pptxBaltazarRosales1
Ã˝
Biomolecules are complex organic molecules essential for life, categorized into carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates serve as energy sources and structural components, proteins facilitate numerous cellular functions and reactions, lipids provide energy storage and cell membrane structure, while nucleic acids contain genetic information and direct protein synthesis. Each class of biomolecules is composed of specific elements and structural units, highlighting their critical roles in biological systems.More Related Content
Similar to electronic configuration.pptx ghjjkgjhjhklh (20)
Pauli exclusion principle
Pauli exclusion principleNaheemPinchoos
Ã˝
Wolfgang Pauli formulated the Pauli Exclusion Principle in 1925, for which he received the Nobel Prize in 1945. The principle states that no two electrons in an atom can have the same set of quantum numbers, meaning each electron must have its own unique spin state. This explains the electron configurations of atoms and the periodic table. The principle is fundamental to quantum mechanics and is important for understanding chemical bonding and the properties of solids.Applied Chemistry, atomic and molecular structure, part 1, by Shiraz mahbob PhD
Applied Chemistry, atomic and molecular structure, part 1, by Shiraz mahbob PhDMaqsoodAhmadKhan5
Ã˝
The document provides an overview of atomic and molecular structure, covering topics such as atomic number, isotopes, and the arrangement of electrons in orbitals. It explains fundamental principles like the Pauli exclusion principle, Hund's rule, and the Aufbau principle in filling atomic orbitals, as well as the periodic table and trends in properties across periods and groups. Additionally, it touches on chemical bonding concepts, including ionic and covalent bonds, polarity, and VSEPR theory for predicting molecular shape.MODULE-1-Quantum-mechanical-Model-of-Atom-Copy.pptx
MODULE-1-Quantum-mechanical-Model-of-Atom-Copy.pptxabajabajajaba21
Ã˝
MODULE-1-Quantum-mechanical-Model-of-Atom-Copy.pptxchemical bonding and molecular structure
chemical bonding and molecular structure Akarshik Banerjee
Ã˝
This document presents information about chemical bonding from the chemistry project of students Akarshik Banerjee, Pratyush Dey, and Sayantan Biswas. It discusses various types of chemical bonds including covalent bonds, which form when atoms share electron pairs, and ionic bonds, which form through complete electron transfer. It also describes concepts like the octet rule, Lewis dot structures, formal charge, resonance structures, and molecular geometry based on valence shell electron pair repulsion theory. Hybridization and molecular orbitals are explained as well. In summary, the document provides an overview of key concepts in chemical bonding from the perspective of high school chemistry students.Qrt-2-Week-1-Module-1-Lesson-1.pptx
Qrt-2-Week-1-Module-1-Lesson-1.pptxMATRIXGAMING8
Ã˝
This document provides an overview of the quantum mechanical model of the atom. It discusses key concepts like the development of atomic models, electron configuration, quantum numbers, electron orbitals, and energy levels. Some key points:
- The quantum mechanical model describes the probable locations of electrons in an atom and how they can have discrete energy levels. It improved upon earlier planetary and Bohr models.
- Electrons occupy specific orbitals and energy levels denoted by quantum numbers like principal and azimuthal quantum numbers.
- Electron configuration notation specifies the distribution of electrons across orbitals based on Aufbau's principle and other rules.
- Transitions between energy levels explain the emission or absorption of photons and thus the colorsModels
ModelsGalen West
Ã˝
The document discusses the evolution of atomic models over time from Dalton's model to the current quantum mechanical model. It summarizes key developments including the plum pudding model, Bohr's model of electrons in orbits, and how the Schrodinger equation led to the quantum mechanical model where electrons occupy distinct energy levels and orbitals. The modern model describes electron probability clouds rather than set orbits and accounts for properties like electron spin and the Pauli exclusion principle.Electron_Energy_Level_Notes.ppt
Electron_Energy_Level_Notes.pptCYRILCONSTANTINO2
Ã˝
1) Electrons in atoms occupy different energy levels rather than following classical orbital models. Higher energy levels are farther from the nucleus.
2) Energy levels are divided into sublevels which have different shapes designated by letters. Electrons fill these sublevels according to specific rules.
3) The aufbau principle and Pauli exclusion principle govern how electrons fill atomic orbitals based on energy and allowing no more than two electrons of opposite spin per orbital. Hund's rule favors occupying each orbital in a sublevel singly before pairing electrons.SCI-9-Quarter-2- electron configuration.pptx
SCI-9-Quarter-2- electron configuration.pptxpauloalegria3
Ã˝
The document discusses electron configuration, which refers to the arrangement of electrons in an atom's energy levels and is vital for understanding atomic properties and interactions. Key principles like the Aufbau principle, Pauli exclusion principle, and Hund's rule govern how electrons are distributed among orbitals. Additionally, the document explains how electron configurations affect reactivity and bonding in elements.Electronic-configuration for last term .pptx
Electronic-configuration for last term .pptxivaratot
Ã˝
The document provides an overview of electron configurations and orbital energy levels in hydrogen and many-electron atoms, emphasizing how energy depends on quantum numbers and various interactions in the atom. It describes ground states, excited states, and the application of principles such as Hund's rule and the Pauli exclusion principle in determining electron arrangements. Additionally, it explains the order of filling atomic orbitals based on the aufbau principle and how these configurations relate to the periodic table.Ch 5 electrons in atoms notes
Ch 5 electrons in atoms notesEsther Herrera
Ã˝
The document discusses the quantum mechanical model of the atom. It explains that electrons can occupy certain allowed energy levels around the nucleus, represented by principal quantum numbers. Within these levels are smaller energy sublevels that determine the shape of an atom's orbitals. Electrons fill these orbitals based on specific rules, resulting in unique electron configurations for each element. The configurations and valence electrons allow atoms to gain or lose energy through emission or absorption of photons in quantized amounts, explaining atomic emission spectra.Test
Testrajashekhar2000
Ã˝
The document discusses the atomic structure and models proposed by scientists over time. It explains that the atom consists of a nucleus and electrons. Rutherford proposed that the nucleus is at the center of the atom and contains protons and neutrons, while electrons orbit the nucleus. Bohr's atomic model built on Planck's quantum theory by postulating that electrons orbit in fixed energy levels and can only absorb or emit photons of energy equal to the difference between orbit levels. The model helped explain atomic stability and emission spectra but had limitations for multi-electron atoms.chemical bonding ##@ Abhay
chemical bonding ##@ AbhayAbhay Shah
Ã˝
This document discusses different chemical bonding theories including:
- Covalent bonding which occurs when atoms share electron pairs
- Ionic bonding which involves a complete transfer of electrons between atoms
- Lewis structures which represent bonding using shared electron pairs and the octet rule
- VSEPR theory which predicts molecular geometry based on electron pair repulsion
- Hybridization which involves mixing of atomic orbitals to form new hybrid orbitals
- Molecular orbital theory where atomic orbitals combine to form molecular orbitals of lower energy, providing stable bonding.QUARTER 2 SCIENCE 9.pptx hhfcdlif97v/ vgjyt
QUARTER 2 SCIENCE 9.pptx hhfcdlif97v/ vgjytjeanmycamontalbo
Ã˝
The document outlines various atomic theories, starting with John Dalton's solid sphere model, which posits that all matter is composed of indivisible atoms that differ by element. It progresses through significant models by Thomson, Rutherford, Bohr, and Schrodinger, each building on the understanding of atomic structure, from the distribution of charges to the electron cloud concept. Additionally, it discusses electron configurations and quantum numbers that define the arrangement and behavior of electrons in an atom.B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...Rai University
Ã˝
The document discusses rules for electron arrangement in orbitals. It covers three main principles:
1) Aufbau's principle states that electrons fill atomic orbitals in order of increasing energy. Electrons occupy the lowest energy orbitals first.
2) Pauli's exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This limits each orbital to holding two electrons with opposite spins.
3) Hund's rule states that when multiple degenerate orbitals are available at the same energy level, electrons will occupy them singly with parallel spins before pairing up. This results in the highest possible spin state.B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...Rai University
Ã˝
This document discusses the rules for electron arrangement in orbitals. It covers three main principles:
1) Aufbau's principle, which states that electrons fill atomic orbitals in order of increasing energy.
2) Pauli's exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. This limits each orbital to holding two electrons with opposite spins.
3) Hund's rule, which specifies that electrons first occupy degenerate orbitals singly with parallel spins before pairing up. This maximizes the multiplicity of electrons in orbitals of equal energy.B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...
B.sc(microbiology and biotechnology and biochemistry) ii inorganic chemistry ...Rai University
Ã˝
This document discusses the rules for electron arrangement in orbitals. It covers three main principles:
1) Aufbau's principle, which states that electrons fill atomic orbitals in order of increasing energy.
2) Pauli's exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. This limits each orbital to holding two electrons with opposite spins.
3) Hund's rule, which specifies that electrons first occupy degenerate orbitals singly with parallel spins before pairing up. This maximizes the multiplicity of electrons in orbitals of equal energy.Lesson 5 - Electronic Configuration.pptx
Lesson 5 - Electronic Configuration.pptxDoveLalune
Ã˝
The document discusses key concepts in atomic structure, including the organization of electrons in energy levels and principles for filling atomic orbitals such as the Aufbau principle, Hund's rule, and the Pauli exclusion principle. It outlines how to write electron configurations and provides examples for various elements. Overall, it serves as a guide to understanding electron distribution in atoms.Quantum Numbers (1).pdf
Quantum Numbers (1).pdfRoxette Rosete
Ã˝
The document summarizes the four quantum numbers that describe electrons: principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). It then discusses electron configuration, which is the arrangement of electrons in an atom based on the Aufbau principle, Pauli exclusion principle, and Hund's rule. Electron configuration can be written using symbols, orbital diagrams, noble gas notation, and electron-dot structures.Electron configuration
Electron configurationMariaisa Apellidos
Ã˝
Electron configuration represents the arrangement of electrons in an atom's orbital shells and subshells. There are three main rules that determine electron configuration: the Aufbau principle, which states that orbitals are filled in order of increasing energy; the Pauli exclusion principle, where no two electrons can have the same quantum numbers; and Hund's rule, where orbitals in a subshell are singly occupied with parallel spins before pairing. Electron configuration can be written out or represented through orbital diagrams, the periodic table, or Möller diagrams.Electron Structure
Electron StructureTrevor23
Ã˝
The document discusses the Bohr model of the hydrogen atom. It explains that the electron can only orbit the nucleus in fixed energy levels called orbits. The lowest energy level is the ground state, with higher levels being excited states. When an electron moves to a higher level, energy is absorbed, and when it moves to a lower level, a photon of energy is released. The wavelength and frequency of light are related to the energy of photons by Planck's constant and the speed of light. Quantum numbers describe the properties of atomic orbitals and electrons, including principal, orbital, and spin quantum numbers. Electron configurations show how electrons are arranged in orbitals according to Aufbau principle, Hund's rule, and Pauli exclusionMore from BaltazarRosales1 (16)
mirrorrs, concave convex. formula solution
mirrorrs, concave convex. formula solutionBaltazarRosales1
Ã˝
The document provides tips to reduce gadget exposure, including setting screen time limits, creating gadget-free zones, and engaging in offline activities. It discusses the effects of various types of radiation, emphasizing that long exposure to harmful radiation can lead to health issues. Additionally, it explains the laws of reflection and characteristics of images formed by plane mirrors.Biomolecules.pptxBiomolecules.pptxBiomolecules.pptx
Biomolecules.pptxBiomolecules.pptxBiomolecules.pptxBaltazarRosales1
Ã˝
Biomolecules are complex organic molecules essential for life, categorized into carbohydrates, proteins, lipids, and nucleic acids. Carbohydrates serve as energy sources and structural components, proteins facilitate numerous cellular functions and reactions, lipids provide energy storage and cell membrane structure, while nucleic acids contain genetic information and direct protein synthesis. Each class of biomolecules is composed of specific elements and structural units, highlighting their critical roles in biological systems.BOYLES LAW.pptx jhghjghghjgjkggghghghghgg
BOYLES LAW.pptx jhghjghghjgjkggghghghghggBaltazarRosales1
Ã˝
Boyle's Law, established by Robert Boyle in 1662, describes the inverse relationship between gas volume and pressure at constant temperature. The document covers essential properties of gases including pressure, volume, and temperature, with standard units and conversion factors provided. Additionally, it includes various theoretical problems and examples demonstrating the application of Boyle's Law in calculating gas behavior under changing conditions.PARTS OF MALE REPRODUCTIVE SYSTEM.pptxvhgg
PARTS OF MALE REPRODUCTIVE SYSTEM.pptxvhggBaltazarRosales1
Ã˝
The document summarizes the major parts of the male reproductive system, including the scrotum, testes, epididymis, vas deferens, ejaculatory duct, urethra, and penis. It describes the development and maturation of sperm cells within the testes and along the epididymis, vas deferens, and ejaculatory duct. It also explains that testosterone is the main male sex hormone responsible for male sexual development and characteristics.OCCURENCE OF EVOLUTION.pptx how does evolution happens
OCCURENCE OF EVOLUTION.pptx how does evolution happensBaltazarRosales1
Ã˝
This document discusses several key concepts related to the occurrence and mechanisms of evolution:
1. It outlines Jean Baptiste de Lamarck and Charles Darwin's influential theories of evolution, including Lamarck's theories of need, use and disuse, and acquired characteristics as well as Darwin's theory of natural selection.
2. It explores the genetic basis of evolution through concepts like population genetics, gene flow, allele frequencies, non-random mating, and the Hardy-Weinberg equilibrium.
3. It examines various evolutionary patterns such as natural selection, genetic drift, speciation, punctuated equilibrium, microevolution, coevolution, convergent evolution, and adaptive radiation.EVIDENCE OF EVOLUTION.pptx history of evolution
EVIDENCE OF EVOLUTION.pptx history of evolutionBaltazarRosales1
Ã˝
This document provides evidence of evolution through figures showing similarities in human and animal anatomy. Figure 6 shows the human digestive system with the appendix. Figure 7 shows a snake pelvis bone with a reduced hind limb. Figures 8 and 8B show that early embryonic stages and advanced embryos of different vertebrates share similar structures, providing evidence they evolved from a common ancestor.NERVOUS SYSTEM.pptx tdtffykgygigguiggugiug
NERVOUS SYSTEM.pptx tdtffykgygigguiggugiugBaltazarRosales1
Ã˝
The nervous system is composed of two main divisions - the central nervous system consisting of the brain and spinal cord, and the peripheral nervous system consisting of nerves that connect to all parts of the body. It coordinates feedback mechanisms through the peripheral and central nervous systems to maintain homeostasis in the body. The nervous system regulates internal processes like temperature, hunger, and organ function to keep the body's conditions stable.MUTATION.pptx learn different ways of mutation how does it happen
MUTATION.pptx learn different ways of mutation how does it happenBaltazarRosales1
Ã˝
The DNA is used to complete protein synthesis which has two stages - transcription and translation. During translation at the ribosome, messenger RNA sequences are read and translated into amino acids which then form proteins. A mutation is a change in the DNA sequence that can occur either from mistakes when DNA copies or environmental factors like UV light and smoking, and takes place during DNA replication which can then cause abnormal transcription into mRNA.PROTIEN SYNTHESIS.pptx help to understand the process of protien synthesis
PROTIEN SYNTHESIS.pptx help to understand the process of protien synthesisBaltazarRosales1
Ã˝
DNA contains genetic codes made up of nucleotide bases that are arranged in triplets to code for amino acids. RNA carries these genetic codes from DNA and uses them to assemble amino acids into proteins according to the code. Together, DNA and RNA work through this process of protein synthesis to produce the proteins specified by the genetic code.festival Dancing and Fitness.pptx........
festival Dancing and Fitness.pptx........BaltazarRosales1
Ã˝
The FITT principle provides an acronym to describe exercise parameters: Frequency refers to the number of weekly sessions; Intensity indicates the difficulty level which can be light, moderate, or vigorous; Time is the duration of each session; and Type specifies the mode of exercise or activity.The Fundamental Body Movements.pptx GFGF
The Fundamental Body Movements.pptx GFGFBaltazarRosales1
Ã˝
Fundamental body movements are the basic building blocks for more complex physical activities like sports and dancing. They include locomotor movements that move the body through space, such as walking, running, and hopping, as well as non-locomotor movements that involve movement of body parts without traveling, like bending, twisting, and swinging. Students must master fundamental movements like dribbling and kicking during early childhood in order to participate in physical activities as they grow older. The document outlines the main categories and examples of fundamental locomotor and non-locomotor movements, as well as elements of rhythm, space, and qualities of movement important for dance.The Endocrine System.pptx GFTFTFYFUYUYYUY
The Endocrine System.pptx GFTFTFYFUYUYYUYBaltazarRosales1
Ã˝
Here are the answers to fill in the blanks:
1. Pituitary gland
2. Thyroid gland
3. Parathyroid gland
4. Parathyroid gland
5. Pancreas
6. Thymus gland
7. Thymus gland
8. Adrenal gland
9. Birth control pills
10. Prolactin and OxytocinMyself on Street and Hip-hop Dances.pptx
Myself on Street and Hip-hop Dances.pptxBaltazarRosales1
Ã˝
1. The document introduces the importance of active recreation for achieving a healthy body and discusses street and hip-hop dances.
2. It provides a series of true/false statements to test the reader's knowledge of concepts like rate of perceived exertion, vigorous vs. passive activities, nutrition, and more.
3. The reader is then asked to identify which physical activities can help sustain fitness by writing "YES" or "NO" next to each one listed, such as watching TV, playing volleyball, eating fruits/veggies, dancing, etc.ARTS 9 L1.pptxHJFJLSKJFKSJGKSDJGKSJJIRJGIJIG
ARTS 9 L1.pptxHJFJLSKJFKSJGKSDJGKSJJIRJGIJIGBaltazarRosales1
Ã˝
This document discusses media-based arts and design in the Philippines. It notes that photography is considered both a tool for communication and an art form. The document outlines key elements of filmmaking such as the director, actors, cinematography, editing, production design. It also briefly mentions notable Philippine photographers and filmmakers.ART NEOCLASSICISM AND ROMANTISMISISM.pptx
ART NEOCLASSICISM AND ROMANTISMISISM.pptxBaltazarRosales1
Ã˝
Neoclassicism and Romanticism were artistic periods between the late 18th and early 19th centuries that had distinct characteristics and elements. Neoclassicism took inspiration from Ancient Greek and Roman art, emphasizing historical accuracy and order. Romanticism was an emotional reaction that celebrated nature and heightened sensations through dramatic compositions. Both periods influenced painting, sculpture, and architecture styles during this time.asexual reproduction ppt.pptx
asexual reproduction ppt.pptxBaltazarRosales1
Ã˝
This document defines asexual reproduction and describes its various types. It explains that asexual reproduction involves a single parent producing offspring that are genetically identical. Various types of asexual reproduction are described, including fission, fragmentation, budding, parthenogenesis, spore production, and vegetative propagation. Examples of each type are provided. The objectives are to define asexual reproduction, describe its different types, and classify organisms by their reproductive methods.Ad
Recently uploaded (20)
GBSN_ Unit 1 - Introduction to Microbiology
GBSN_ Unit 1 - Introduction to MicrobiologyAreesha Ahmad
Ã˝
Microbiology for Nursing students - According to New PNC course curriculum - 2025Role of Glutamate, glutamine and Alanine in Transport of Ammonia in Tissues
Role of Glutamate, glutamine and Alanine in Transport of Ammonia in TissuesTayyab
Ã˝
This slide explains the roles of Glutamate, Glutamine, and Alanine in safely transporting toxic ammonia from tissues to the liver and kidneys, where it is detoxified or excreted."Deconstruction Analysis The adventure of devil's foot by Sir Arthur Conan Doyle
Deconstruction Analysis The adventure of devil's foot by Sir Arthur Conan Doylestaverechard
Ã˝
Arthur Conan Doyle (1859-1930) was a Scottish writer and physician who is widely recognized as the creator of the legendary detective character, Sherlock Holmes. Born in Edinburgh, Doyle studied medicine at the University of Edinburgh, where he began writing short stories in his spare time. Although he initially worked as a doctor, his name became widely known when he published the Sherlock Holmes stories, starting with A Study in Scarlet (1887). (Sakula,1997). Sherlock Holmes and Dr. Watson are on holiday in Cornwall, seeking rest for Holmes. They become involved in a mysterious case involving the deaths and madness of the Tregennis family. One sister dies with a terrified expression, while the two brothers are found insane. Holmes discovers that a rare West African poison called "Devil’s Foot" was used, which affects the mind. The murderer is ultimately revealed to be Dr. Leon Sterndale, an explorer and relative of the family, who takes justice into his own hands. Synthesis and characterization of Thiazole derivatives of N-substituted lsatin
Synthesis and characterization of Thiazole derivatives of N-substituted lsatinProfessional Content Writing's
Ã˝
Thiazole derivatives of N-substituted isatin have attracted significant interest due to their wide-ranging applications in medicinal chemistry, pharmaceuticals, and materials science. These compounds exhibit diverse biological activities, making them promising drug candidates, while their unique chemical structures offer potential in designing advanced functional materials. This presentation focuses on the synthesis and characterization of these derivatives through targeted chemical reactions involving various substituents on the isatin and thiazole cores, enabling the fine-tuning of their biological and physical properties. Characterization techniques such as NMR, FT-IR, Mass Spectrometry, and X-ray crystallography are employed to confirm molecular structures and analyze solid-state properties. These methods provide critical insights into the structure–activity relationships of the synthesized compounds. Our presentation highlights the synthetic pathways, structural elucidation, and potential applications of thiazole-based N-substituted isatin derivatives, aiming to support ongoing advancements in drug discovery and material development.
About Author:
Noor Zulfiqar is an award-winning chemist, Premium member of American Chemical Society (ACS), certified publisher & peer reviewer, and an experienced academic lecturer. As a professional content creator, she offers top-tier presentation design, research writing, and scientific content development services. Her multidisciplinary expertise spans computational science, chemistry, nanotechnology, environmental studies, socio-economics, human resource management, life sciences, engineering management, medical and pharmaceutical sciences, and business, her work ensures clarity, creativity, and academic excellence. Her services are ideal for those seeking impactful, visually compelling content tailored to diverse academic and research needs.
For collaborations or custom-designed academic content, feel free to reach out!
Contact:
Email: professionalwriter94@outlook.com
Facebook: https://www.facebook.com/share/1LgwpoyLDg/
Website: https://professional-content-writings.jimdosite.comOperationalising OGC Processes with Application Packages in ILIAD: A Service ...
Operationalising OGC Processes with Application Packages in ILIAD: A Service ...Marco Amaro Oliveira
Ã˝
This contribution presents the integration of the EO Application Package model into the ILIAD Digital Twin of the Ocean architecture, using the OGC API Processes DRU specification. Built on the EOEPCA framework and OGC best practices and specifications, the approach enables standardized, containerized EO applications packaged with CWL to run across a wide range of infrastructures including Kubernetes and HPC. These applications are already in use across several platforms, and in ILIAD they have been applied to models such as oil spill forecasting, aquaculture, wave energy, and ship routing.
The EDITO platform supports OGC API Processes but is currently optimized for simpler workflows using environment variables. To enhance compatibility with EO Application Packages, ILIAD introduces a Kubernetes-based ADES implementation, enabling dynamic execution and integration with EDITO's object store and metadata catalog. The experience is also informing the evolution of the OGC Best Practice, and practical solutions for bridging architectural gaps will be discussed.Citizen Science and Science communication
Citizen Science and Science communicationtarhanhatice0101
Ã˝
Science communication using citizen scientistsGBSN__Unit 2 - Control of Microorganisms
GBSN__Unit 2 - Control of MicroorganismsAreesha Ahmad
Ã˝
Microbiology for Nursing students - According to New PNC course curriculum - 2025
TISSUE TRANSPLANTATTION and IT'S IMPORTANCE IS DISCUSSED
TISSUE TRANSPLANTATTION and IT'S IMPORTANCE IS DISCUSSEDPhoebeAkinyi1
Ã˝
Tissue transplant in immunologyMOLD -GENERAL CHARACTERISTICS AND CLASSIFICATION
MOLD -GENERAL CHARACTERISTICS AND CLASSIFICATIONaparnamp966
Ã˝
This is a brief note on types of organism on food with special focus on molds. This includes their general characteristics, spores; their classification, mycotoxins, and how the molds affect food. HOW TO FACE THREATS FROM THE FORCES OF NATURE EXISTING ON PLANET EARTH.pdf
HOW TO FACE THREATS FROM THE FORCES OF NATURE EXISTING ON PLANET EARTH.pdfFaga1939
Ã˝
This article aims to present how to deal with localized or global threats to human beings caused by the forces of nature that exist on planet Earth. The threats to human beings caused by the forces of nature that exist on planet Earth include earthquakes and tsunamis that can affect specific areas of the planet, volcanic eruptions that can affect specific areas of the planet or have a global impact, the cooling of the Earth's core and the inversion of the Earth's magnetic poles, both of which have a global impact. Specific measures are proposed to deal with each of the threats, but in addition to these, it is necessary to create a global structure, a World Organization for Defense Against Natural Disasters with global scope linked to the UN (United Nations) that has the capacity to technically coordinate the actions of countries around the world in dealing with these threats. The creation of this body is absolutely necessary because most of the threats from the forces of nature that exist on planet Earth have a global impact. To carry out its functions, this body must have financial resources from a global fund against natural disasters of global scope to be maintained by all countries on the planet and administered by the UN.Pneumonia Presentation for CPG Review and Mastery
Pneumonia Presentation for CPG Review and MasteryJayricDepalobos
Ã˝
Pneumonia Presentation for Internal Medicine DepartmentSynthesis and characterization of Thiazole derivatives of N-substituted lsatin
Synthesis and characterization of Thiazole derivatives of N-substituted lsatinProfessional Content Writing's
Ã˝
Operationalising OGC Processes with Application Packages in ILIAD: A Service ...
Operationalising OGC Processes with Application Packages in ILIAD: A Service ...Marco Amaro Oliveira
Ã˝
Ad
electronic configuration.pptx ghjjkgjhjhklh
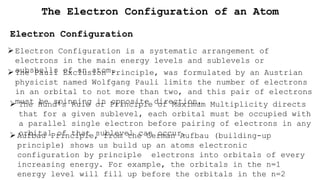
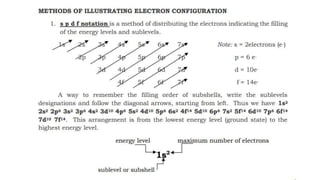
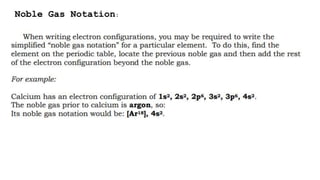
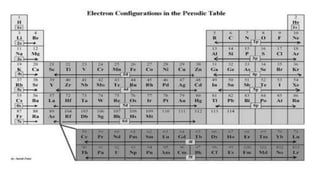
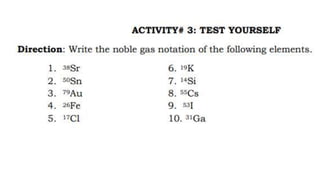
- 1. The Electron Configuration of an Atom Electron Configuration Electron Configuration is a systematic arrangement of electrons in the main energy levels and sublevels or subshells of an atom. The Pauli Exclusion Principle, was formulated by an Austrian physicist named Wolfgang Pauli limits the number of electrons in an orbital to not more than two, and this pair of electrons must be spinning in opposite direction,  The Hund’s Rule or Principle of Maximum Multiplicity directs that for a given sublevel, each orbital must be occupied with a parallel single electron before pairing of electrons in any orbital of that sublevel can occur. Aufbau Principle, from the German Aufbau (building-up principle) shows us build up an atoms electronic configuration by principle electrons into orbitals of every increasing energy. For example, the orbitals in the n=1 energy level will fill up before the orbitals in the n=2