Lez05 spettroscopia x
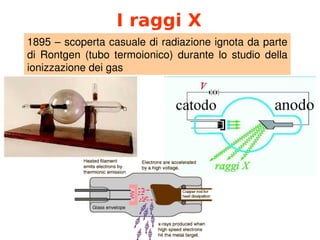
- 1. I raggi X 1895╠²ŌĆō╠²scoperta╠²casuale╠²di╠²radiazione╠²ignota╠²da╠²parte╠² di╠² Rontgen╠² (tubo╠² termoionico)╠² durante╠² lo╠² studio╠² della╠² ionizzazione╠²dei╠²gas╠²
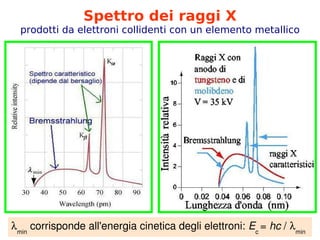
- 2. Spettro dei raggi X prodotti da elettroni collidenti con un elemento metallico ’ü¼min ╠²corrisponde╠²all'energia╠²cinetica╠²degli╠²elettroni:╠²Ec╠² =╠²hc╠²/╠²’ü¼min
- 3. Diagramma dei livelli atomici La╠² fastidiosa╠² notazione╠² spettroscopica╠²dei╠²raggi╠²X: Le╠² transizioni╠² atomiche╠² che╠² originano╠² lo╠² spettro╠² caratteristico╠² dei╠² raggi╠² X╠² si╠² indicano╠²con╠²nomi╠²speciali: Righe╠² K: ╠²lo╠² stato╠² finale╠² dell'elettrone╠² che╠² decade╠² ├©╠² il╠² livello╠² K,╠² cio├©╠² n=1, ╠²(K’üĪ ╠²se╠² il╠² livello╠²iniziale╠²├©╠²L,╠²K’üó ╠²se╠²├©╠²M,ŌĆ”) Righe╠²L:╠²lo╠²stato╠²finale╠²├©╠²il╠²livello╠² L,╠² cio├©╠² n=2, ╠²(L’üĪ ╠²se╠² il╠² livello╠² iniziale╠²├©╠²M,╠²L’üó ╠²se╠²├©╠²N,ŌĆ”)diagramma╠²livelli╠²X di╠²Mo╠²(Z=42)
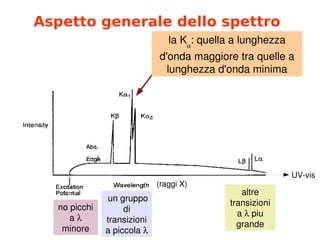
- 4. Aspetto generale dello spettro la╠²K’üĪ :╠²quella╠²a╠²lunghezza╠² d'onda╠²maggiore╠²tra╠²quelle╠²a╠² lunghezza╠²d'onda╠²minima ╠²un╠²gruppo╠² di╠² transizioni╠² a╠²piccola╠²’ü¼ no╠²picchi╠² a╠²’ü¼╠² minore altre╠² transizioni╠² a╠²’ü¼’ĆĀpiu╠² grande UV┬Łvis (raggi╠²X)
- 5. Legge di Moseley ŌĆó╠² Nel╠² 1913╠² Moseley╠² genera╠² gli╠² spettri╠² caratteristici╠² di╠² tutti╠² gli╠² elementi╠²allora╠²noti ŌĆó╠²La╠²lunghezza╠²d'onda╠²’ü¼ (o╠² equivalentemente╠² la╠² frequenza╠² ’ü« ╠²delle╠² righe╠² K’üĪ ╠²di╠² elementi╠² diversi╠² dipendono╠² solo╠² dal╠² numero╠² atomico╠² Z ╠²e╠² obbediscono╠² a╠² una╠² legge╠² semplice╠² (e╠² cos├¼╠² fanno╠²tutte╠²le╠²altre╠²righe) Z
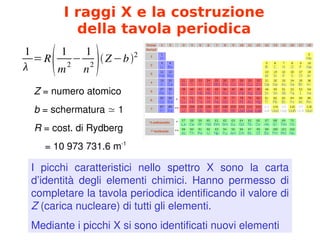
- 6. I raggi X e la costruzione della tavola periodica 1 ╬╗ =R Ņé× 1 m2 ŌłÆ 1 n2 Ņé¤Ņé×ZŌłÆbŅé¤2 Z╠²=╠²numero╠²atomico b╠²=╠²schermatura╠²Ōēā╠²1 R╠²=╠²cost.╠²di╠²Rydberg ╠²╠²╠²╠²=╠²10╠²973╠²731.6╠²m┬Ł1 I╠² picchi╠² caratteristici╠² nello╠² spettro╠² X╠² sono╠² la╠² carta╠² dŌĆÖidentit├Ā╠² degli╠² elementi╠² chimici.╠² Hanno╠² permesso╠² di╠² completare╠²la╠²tavola╠²periodica╠²identificando╠²il╠²valore╠²di╠² Z╠²(carica╠²nucleare)╠²di╠²tutti╠²gli╠²elementi. Mediante╠²i╠²picchi╠²X╠²si╠²sono╠²identificati╠²nuovi╠²elementi
- 7. Confronto spettro ottico - raggi X Raggi╠²X╠² ŌĆólivelli╠²pi├╣╠²interni╠²(di╠²core) ŌĆófrequenze/energie╠² elevate╠² (h’ü«╠²>╠²50╠²eV) ŌĆópoco╠² influenzati╠² dai╠² legami╠² chimici╠²╠² ŌĆópoche╠²righe╠²ben╠²identificate ŌĆóEccitazione: ╠²si╠² crea╠² una╠² lacuna╠² (hole)╠² in╠² un╠² livello╠² profondo╠² (di╠² core)╠² portando╠² l'elettrone╠²in╠²un╠²livello╠²vuoto Spettri╠²ottici ŌĆólivelli╠²pi├╣╠²esterni╠²(valenza) ŌĆófrequenze/energie╠² ottiche╠² (1╠²eV╠²<╠²h’ü«╠²<╠²20╠²eV) ŌĆócambiano╠² molto╠² nelle╠² molecole╠²rispetto╠²agli╠²atomi ŌĆótantissime╠²righe ŌĆóEccitazione: ╠²si╠² promuove╠² un╠² elettrone╠² di╠² valenza╠² ad╠² un╠²livello╠²vuoto╠²pi├╣╠²alto
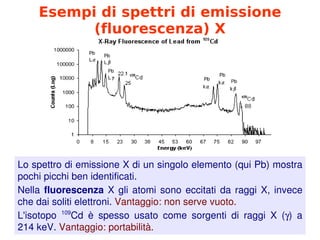
- 8. Esempi di spettri di emissione (fluorescenza) X Lo╠²spettro╠²di╠²emissione╠²X╠²di╠²un╠²singolo╠²elemento╠²(qui╠²Pb)╠²mostra╠² pochi╠²picchi╠²ben╠²identificati. Nella╠²fluorescenza╠²X╠²gli╠²atomi╠²sono╠²eccitati╠²da╠²raggi╠²X,╠²invece╠² che╠²dai╠²soliti╠²elettroni.╠²Vantaggio:╠²non╠²serve╠²vuoto. L'isotopo╠² 109 Cd╠² ├© ╠²spesso╠² usato╠² come╠² sorgenti╠² di╠² raggi╠² X╠² (’ü¦)╠² a╠² 214╠²keV.╠²Vantaggio:╠²portabilit├Ā.
- 9. Esempi di spettri di fluorescenza X I╠²picchi╠²caratteristici╠²di╠²vari╠²elementi╠²si╠²distinguono╠²chiaramente╠² nello╠²spettro╠²di╠²emissione:╠²un╠²ottimo╠²tool╠²analitico.
- 12. Confronto spettro ottico - raggi X: differenze tecnologiche Spettroscopia╠²X╠² ŌĆóvuoto ŌĆómonocromatori╠²a╠²cristalli╠²(Si) ŌĆósorgenti:╠² anodo╠² rotante,╠² decadimenti╠² nucleari╠² (109 Cd),╠² sincrotroni ŌĆólenti╠² inefficienti╠² (n ╠²~ ╠²1:╠² solo╠² Fresnel),╠² specchi╠² inefficienti╠² (solo╠² cristalli,╠² di╠² solito╠² Si)╠² e╠² ingombranti,╠² fenditure╠² scatterano╠² e╠² emettono╠² elettroni,╠²ŌĆ£finestreŌĆØ╠²inefficienti╠² di╠²Be. Spettroscopia╠²ottica ŌĆóaria ŌĆómonocromatori╠²a╠²reticolo ŌĆóluminose╠² &╠² efficienti╠² sorgenti╠² standard╠² (lampade/laser) ŌĆólenti,╠² specchi,╠² fenditure,╠² ŌĆ£finestreŌĆØ╠² semplici,╠² standard,╠² economici╠² &╠² efficienti
- 13. Assorbimento di raggi X Interazione╠²raggi╠²X┬Ł╠²materia Cessione╠²di╠²energia╠²soprattutto╠²agli╠²elettroni╠²╠² interni,╠²con╠²possibile╠²eccitazione╠²degli╠²atomi Atomo╠² eccitato╠² diventa╠² instabile╠² e╠² poi╠² decade... Due╠²processi╠²importanti:Due╠²processi╠²importanti: EffettoEffetto fotoelettricofotoelettrico (elettroni interni) EffettoEffetto ComptonCompton (elettroni valenza) 3p 3s 1s 2p 2s 4s 3d K L M 4p
- 14. Effetto fotoelettrico fotone╠²X╠²di╠²bassa╠²energia╠²interagisce╠²con╠²elettrone╠²legato╠² cedendo╠²tutta╠²la╠²sua╠²energia Step╠² 1:╠² un╠² elettrone╠² di╠² core╠² ├© espulso╠²e╠²si╠²forma╠²una╠²lacuna Step╠²2:╠²un╠²elettrone╠²da╠²salta╠²da╠² un'orbita╠² pi├╣╠² alta╠² a╠² colmare╠² la╠² lacuna,╠² con╠² emissione╠² X╠² caratteristica Possibilit├Ā╠² di╠² nuovo╠² effetto╠² fotoelettrico:╠² si╠² crea╠² possibilit├Ā╠² di╠² reazione╠² ŌĆ£a╠² catenaŌĆØ╠² (anche╠² l'elettrone╠² espulso╠²pu├▓╠²ionizzare) Eccitazioni╠² elettroniche╠² ad╠² alta╠² energia:╠² danni╠² biologici╠² se╠² il╠² materiale╠² bersaglio╠² consiste╠² di╠² cellule
- 15. Spettri╠²caratteristici╠²degli╠²atomi,╠²semplice╠²dipendenza╠²da╠²Z. Scarsa╠²dipendenza╠²dall'environment╠²chimico.Scarsa╠²dipendenza╠²dall'environment╠²chimico. Spettri di assorbimento di raggi X elettrone╠²strappato╠² da╠²shell╠²K╠²(n=1) elettrone╠²strappato╠² da╠²shell╠²L╠²(n=2) L'assorbimento╠²totale╠² tende╠²ad╠²aumentare╠²con╠²Z
- 16. Effetto Compton Radiazioni╠² di╠² alta╠² energia.╠² Il╠² fotone╠² cede╠² parte della╠²sua╠²energia╠²ad╠²elettroni╠²pi├╣╠²esterni╠²(ŌĆ£liberiŌĆØ) Il╠² fotone╠² diffuso╠² pu├▓╠² interagire╠²di╠²nuovo╠²con╠² effetto╠² fotoelettrico╠² o╠² Compton Spettro╠²continuo L'intensit├Ā╠²di╠²effetto╠²fotoelettrico╠²e╠²Compton╠²aumenta╠²con╠²Z La╠² maggior╠² parte╠² dei╠² materiali╠² biologici╠² sono╠² composti╠² soprattutto╠² di╠² atomi╠² a╠² basso╠² Z ╠²(H╠² (Z=1),╠² C╠² (Z=6),╠² N╠² (Z=7),╠² O╠² (Z=8):╠²Compton╠²domina╠²a╠²Efotone ╠²>╠²300╠²keV
- 17. Legge dellŌĆÖassorbimento I╠²=╠²intensit├Ā╠²finale╠²dopo╠²spessore╠²x╠²[W/m2 ] I0=╠²intensit├Ā╠²iniziale╠²[W/m2 ] x╠²=╠²spessore╠²materiale╠²attraversato╠²[m] ’üŁ’ĆĀ=╠²coefficiente╠²lineare╠²di╠²assorbimento╠²[m┬Ł1 ] I=I0 eŌłÆ╬╝ x I╠² due╠² effetti╠² implicano╠² cessione╠² di╠² energia╠² alla╠² materia╠² con╠² diminuzione╠²di╠²intensit├Ā╠²della╠²radiazione╠²incidente Corpo╠²di╠²vertebrati:╠²la╠²radiazione╠²├©╠²assorbita╠²diversamente╠²dai╠² diversi╠²tessuti╠²che╠²lo╠²compongono.╠²Ad╠²es.╠²le╠²ossa╠²contengono╠² molto╠² calcio╠² (Z╠² =╠² 20)╠² e╠² producono╠² quindi╠² maggior╠² assorbimento╠²(’üŁ╠²maggiore)╠²che╠²i╠²muscoli╠²(Z╠²Ōēā╠²6) ’üŁ╠²grande╠²=╠²breve╠²percorso╠²della╠²radiazione ’üŁ╠²piccolo╠²=╠²percorso╠²della╠²radiazione╠²lungo
- 18. Applicazioni: Radiografia La╠² pellicola╠² registra╠² ombreombre ╠²pi├╣╠² o╠² meno╠² intense╠² in╠² un╠² fascio╠² circa╠² parallelo╠² di╠² raggi╠² X.╠² Tradizionalmente,╠² le╠² immagine╠² sono╠² registrate╠² con╠² pellicola╠² fotografica╠²negativa: Zone╠²pi├╣╠²chiare:╠²intensit├Ā╠²raggi╠²X╠² ├©╠² minore╠² (tessuti╠² con╠² maggiore╠² assorbimento) Zone╠²pi├╣╠²scure:╠²intensit├Ā╠²raggi╠²X╠² ├©╠² maggiore╠² (tessuti╠² con╠² debole╠² assorbimento)
- 19. Tomografia a scansione parallela
- 20. Tomografia assiale computerizzata (TAC, o CAT ŌĆō CT in inglese)
- 21. Microscopia X Micro┬Łtomografia╠²a╠²raggi╠²X╠²di╠²una╠² cellula╠² di╠² lievito.╠² Sono╠² visibili╠² gli╠² organelli╠²interni.╠²Rosso:╠²il╠²nucleo╠²e╠² un╠²grosso╠²vacuolo.╠²Bianco:╠²gocce╠² di╠²lipidi.╠²Arancione/verde:╠²strutture╠² citoplasmiche. http://www.lbl.gov/Science┬ŁArticles/Archive/ALS┬Łx┬Łray┬Łmicroscopy.html Microimmagine╠² a╠² raggi╠² X╠² di╠² materiali╠² biologici╠² in╠² un╠² minerale,╠² con╠² analisi╠² di╠² elementi╠² mediante╠² fluorescenza. http://www.esrf.eu/UsersAndScience/Publications/Highlights/2007/XIM/XIM8 Vantaggio su luce visibile: ’ü¼ minore ŌćÆ meno diffrazione, pi├╣ dettaglio
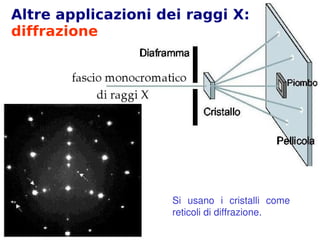
- 22. Altre applicazioni dei raggi X: diffrazione Si╠² usano╠² i╠² cristalli╠² come╠² reticoli╠²di╠²diffrazione.
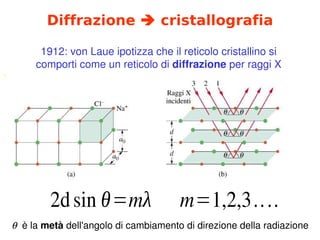
- 23. Diffrazione ŅÜĢ cristallografia ` 1912:╠²von╠²Laue╠²ipotizza╠²che╠²il╠²reticolo╠²cristallino╠²si╠² comporti╠²come╠²un╠²reticolo╠²di╠²diffrazione╠²per╠²raggi╠²X 2dsin ╬Ė=m╬╗ m=1,2,3.... ’ü▒╠²╠²├©╠²la╠²met├Ā╠²dell'angolo╠²di╠²cambiamento╠²di╠²direzione╠²della╠²radiazione
- 24. Diffrazione da cristalli semplici SiC,╠²polvere╠²o╠²policristallo Si╠²(direz.╠²111),╠²monocristallo 2dsin ╬Ė=m╬╗ m=1,2,3....
- 25. Struttura delle macromolecole Proteine╠²(es.╠²enzimi)╠²e╠²DNA/RNA╠² (es.╠²virus)╠²sono╠²molecole╠²formate╠² da╠² migliaia╠² di╠² atomi,╠² e╠² formano╠² cristalli╠² molto╠² complicati,╠² composti╠²principalmente╠²di╠²H,╠²C,╠² N,╠²O╠²(atomi╠²biologici,╠²basso╠²Z). Tecnica╠² degli╠² atomi╠² pesanti:╠² atomi╠² ad╠² alto╠² Z ╠²diffondono╠² maggiormente╠² i╠² raggi╠² X.╠² Atomi╠² pesanti╠² (es.╠² Os,╠² Pt,╠² Au,╠² Hg,╠² Pb)╠² vengono╠²usati╠²come╠²marcatori╠²in╠² punti╠²caratteristici╠²della╠²molecola. Studio╠² della╠² differenza╠² delle╠² figure╠² di╠² diffrazione╠² con╠² e╠² senza╠² marcatori╠² d├Ā╠² informazioni╠² sulla╠² struttura╠²delle╠²macromolecole. Pattern╠² di╠² diffrazione╠² e╠² struttura╠² tridimensionale╠² di╠² lac╠² repressor
- 26. Diffrazione dalla doppia elica Il╠² pattern╠² di╠² diffrazione╠² originale╠²che╠²ha╠²permesso╠² di╠²costruire╠²il╠²modello╠²della╠² doppia╠²elica. Maurice╠²Wilkins╠²&╠²Rosalind╠²Franklin esperimenti╠²nel╠²1950╠²┬Ł╠²52 Watson,╠²Crick,╠²and╠²Wilson:╠²Nobel╠²Prize╠²1962












![Legge dellŌĆÖassorbimento
I╠²=╠²intensit├Ā╠²finale╠²dopo╠²spessore╠²x╠²[W/m2
]
I0=╠²intensit├Ā╠²iniziale╠²[W/m2
]
x╠²=╠²spessore╠²materiale╠²attraversato╠²[m]
’üŁ’ĆĀ=╠²coefficiente╠²lineare╠²di╠²assorbimento╠²[m┬Ł1
]
I=I0 eŌłÆ╬╝ x
I╠² due╠² effetti╠² implicano╠² cessione╠² di╠² energia╠² alla╠² materia╠² con╠²
diminuzione╠²di╠²intensit├Ā╠²della╠²radiazione╠²incidente
Corpo╠²di╠²vertebrati:╠²la╠²radiazione╠²├©╠²assorbita╠²diversamente╠²dai╠²
diversi╠²tessuti╠²che╠²lo╠²compongono.╠²Ad╠²es.╠²le╠²ossa╠²contengono╠²
molto╠² calcio╠² (Z╠² =╠² 20)╠² e╠² producono╠² quindi╠² maggior╠²
assorbimento╠²(’üŁ╠²maggiore)╠²che╠²i╠²muscoli╠²(Z╠²Ōēā╠²6)
’üŁ╠²grande╠²=╠²breve╠²percorso╠²della╠²radiazione
’üŁ╠²piccolo╠²=╠²percorso╠²della╠²radiazione╠²lungo](https://image.slidesharecdn.com/lez05spettroscopiax-180407041404/85/Lez05-spettroscopia-x-17-320.jpg)
























































![[Claudia.tomasini.chimica organica m-z_2013-2014]lucidi 2014 parte 6](https://cdn.slidesharecdn.com/ss_thumbnails/claudia-180504211202-thumbnail.jpg?width=560&fit=bounds)
![[Claudia.tomasini.chimica organica m-z_2013-2014]lucidi 2014 iii parte](https://cdn.slidesharecdn.com/ss_thumbnails/claudia-180428083435-thumbnail.jpg?width=560&fit=bounds)



