Hbv
- 1. –ì–µ–ø–∞—Ç–∏—Ç –í –≤–∏—Ä—É—Å –ê–£–°-324: –ë.–ë–∞–¥–∞–º—Ü—ç—Ü—ç–≥, –ë.–°–∞—Ä—É—É–ª–∂–∞–≤—Ö–ª–∞–Ω
- 2. Бүтэц • Дейны биенцэр нь 42 нм хэмжээтэй • Гадна бүрхүүл нь 7-8 нм зузаантай,хоёр давхар липидээс тогтсон гадаргуугийн эсрэгтөрөгч буюу HBsAg(HB surface antigen)нь гурван өөр бүтэц бүхий нуклеотидээс тогтдог.  Том молекулт LHBsAg(L-large)  Дунд молекулт MHBsAg(M-medium)  Жижиг молекулт SHBsAg(s-small) • Капсид нь 22-25нм диаметртэй тэгш хэмт бүтэцтэй
- 3. Бүтэц • preS1,preS2-HBV гадна байрладаг уураг • S-гадаргуугийн уураг • HBс-бөөмийн уураг • ДНХ-ийн молекул • ТР-терминал протейн • RT-эргэх(урвуу транскритаци) • LHBs,SHBs,MNBs- антигений гурван өөр нуклеотид
- 4. НВ вирусын геном • 320 bp урттай ДНХ-ийн хос утаслаг нуклеотид • ДНХ-ийн хос утаслаг нь:  Бүтэн цагираг буюу сөрөг утгат(negativ-minusstrang-) нуклеотид.5` төгсгөлд ковалент холбоо бүхий Р-уургийн молекултай.  Тал цашираг буюу эерэг утгат (positiv-plusstrange+) нуклеотидээс тогтоно.3` төгсгөлд ковалент бус холбоот Р-уургийн молекултай.
- 5. • Гепатит В вирусийн геном нь давтагдан шууд хуулбарлагдах секвенс(direct repeats DR1 DR2)-ийн хэсэгтэй • Секвенс нь тус бүрдээ 11bp урт нуклеотидтэй. • HBV-ын геномд давтагдан хуулбарлагдах чөлөөт дараалал өөр хоорондоо адилгүй тусгаар хэсгүүдийг хуулбарлан нийлэгжүүлэхэд оролцоно. 1. Вирусын гадаргуугын уураг болох SHBsAg,MHBsAg,LHBsAg антиген кодлогч чөлөөт дараалал 2. Капсид болон бөөмийн уураг мөн HBcAg антиген эдгээрт нягт холбогдсон HBeAg антиген кодлогч чөлөөт дараалал 3. Р уураг задлагч чөлөөт дараалал 4. Х-уураг буюу хавдрын уураг кодлогч чөлөөт дараалал нуклеотидын 4 дараалал нь бие биенээсээ үл хамааран тодорхой хэсэг нуклотидыг кодлодог учраас давтагдан хуулбарлагдах чөлөөт дарааллыг нийтэд нь вирусын геном гэнэ.
- 6. Гепатитийн В вирусын геномын тогтоц • ORF-2 хэрчим Pre- S1,Pre-S2,S геном • ORF-1 хэрчимд капсидийн буюу Р геном • ORF-3 хэрчимд С геном • ORF-4 хэрчимд Х геном
- 7. Гепатитын В вирусын бөөмийн антиген (HBcAg) • Гепатитын В вирусын капсидэд бөөмийн антигений уураг агуулагдана. • 22kD молекул жинтэй. • Вирусын репликацын явцад элэгний эсийн киназа ферментийн үйлчлэлээр серин фосфоржих процессыг эрчимжүүлдэг. • Онцлог нь элэгний эсийн уургийн бүлэгтэй холбогдон,элэгний эсийн удмын мэдээллээр хуулбарлагдан зөөвөрлөгдөх уургыг вирусын бүтцийн уураг үүсэх эх болгон хувиргаж вирион бүрэлдэх процесст оролцдог.
- 8. Гепатитын В вирусын эрт илрэх антиген(HBeAg) • HBcAg антигений аминхүчил синтезлэгдэх явцад амин төгсгөлийн хэсэгт 29 аминхүчил нь HBeAg синтезлэгдэх эхлэл болон хувирдаг. • HBeAg антиген синтезлэгдэх эхлэл болсон pre C хэсэг бол тогтвортой сигналпептид бөгөөд элэгний эсийн ретикулийн эндоплазмийн мембраны гадаргууд уураг синтезлэгдэн уртсах процессыг нөхцөлдүүлнэ. • Синтезлэгдсэн уураг Гольджын аппаратыг дамжин элэгний эсийн гадаргууд зөөвөрлөгдөж е-антигенийг тодорхойлно.
- 9. Гепатитын В вирусын гадаргуугын антиген HBsAg • Ортогепадна вирусын гадна бүрхүүлийн мембранд бүтцээрээ ялгаатай гурван өвөрмөц гликопротейн агуулагдана.Үүнийг гепатитын В вирусын гадаргуугын антиген HBsAg гэнэ. • HBsAg-антигенийг  Жижиг молекулт антиген (SHBsAg)  Дунд зэргийн молекулт антиген(MHBsAg)  Том молекулт антиген (LHBsAg) хэмээн нэрлэнэ.
- 10. SHBsAg • В вирусын халдварын үндсэн бүрдэл • 226 аминхүчлээс бүрэлдсэн 24kD (p24) молекул жинтэй антиген. • Аминхүчлийн 146 дэх байрладах аспарагиний үлдэгдэл (Asn146)глюкозижиж 27кD(gp27)молекул жинтэй гликопротейн болдог.
- 11. MHBsAg • 33кD молекул жинтэй хэсгийг pre S2-HBsAg гэж нэрлэдэг.
- 12. LHBsAg • 39kD молекул жинтэй хэсгийг pre S1-HBsAg гэж нэрлэнэ.
- 13. Полимераза(P уураг) • НВ-вирусын ДНХ-ийн тал цагираг • Эерэг утгат нуклеотидын 3` төгсгөлд ковалент бус холбоонд байрлах • 90кD молекул жинтэй • Р-уураг вирусын халдварын нэгж болдог.
- 14. НВх-уураг • 17kD молекул жинтэй гомодимер бүтэцтэй • Ортогепадна вирусын төрөлд илэрдэг. • Элэгний хучуур эсийн хавдар (Hepato zellular karzinom) үүсэх шалтгаан болно. • НВх-уураг элэгний эсийн промотер болон вирусын промотрыг дам идэвхжүүлэгч (transaktivator) болдог.
- 15. • Аденинмонофосфатын циклыг зохицуулагч уургийн трансактиваторын (CREB) хэсэгтэй нэгдэн ТАТА боксын уурагт (TBP) промотерыг идэвхжүүлдэг.ТВР идэвхжихэд НВх уураг өөрийн карбоксил төгсгөлийн доменаар хавдар дарангуйлагч уураг р53-тай холбогдоход эсийн трансактиватор үйлчлэл саатна.Хэрэв бүтэц нь хувирч өөрчлөгдсөн ДНХ-ийн синтез реперацийн мех-аар засагдахгүй бол р53 уураг эмгэг ДНХ агуулсан эсийн апоптозыг хурдасгана.Энэ үед элэгний эсийн хуваагдлын зохицуулга алдагдаж эс хяналтгүй олширсноос элэгний хавдар үүснэ.
- 16. –¢–∞—Ä—Ö–∞–ª—Ç
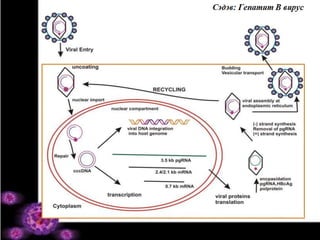
- 17. HBV репликаци: Эзэн биед нэвтрэх • Эзэн биед нэвтрэх: – Парэнтераль: судсаар тариа хийх, эрүүл мэндийн ажилчид өртөмтгий – Бэлгийн зам: биеэ үнэлэгч, ижил хүйстнүүд өртөмтгий – Перинаталь (вертикаль): ээжид нь (HBeAg+) → хүүхэд өртөмтгий
- 18. HBV репликаци: Тархах • Цусаар дамжих – Судасны эндотель • Диссийн зай (Space of Disse) – ЭЛЭГНИЙ ГЕПАТОЦИТ
- 19. HBV репликаци: Элэгний эстэй холбогдох • Гепатоцитын олон төрлийн рецептортой холбогдож болох юм гэж үзэж байна. – LHBsAg (preS1) Гепарин сульфат- протеогликан (муристин) – Липидийн лиганд Холестролын рецептор – Лиганд • Серин протеаза саатуулагч фактор • Ацилгликопротейн • Аннексин V
- 20. HBV репликаци: Эс дотор орох • Эсийн гадна бүрхүүлээ хаях – Капсидаа задлах (элэгний эсийн уургийн оролцоотой) • Чөлөөт геном бичил гуурсаар дамжиж – Бөөмөнд Импортин α, β рецептортой холбогдох • rcDNA (тал цагират, эерэгт утгат) – cccDNA (covalently closed circular DNA) үүснэ. (эписом ба интеграцилагдсан)
- 21. HBV репликаци: Генийн транскрипци • РНХ полимераза II – Pre C → pgRNA (pregenomic бөөмийн урьдал) - 2,4/2,1kb mRNA - 0,7kb mRNA • HBeAg, HBcAg • Вирион цогцолборлохыг дэмжих уургууд • LHBsAg • MHBsAg • SHBsAg • HBxAg • P уураг
- 22. • Тодорхой нэгэн байрлалд зэрэгцэхийг реплиактив • HBxAg элэгний эсийн амин хүчилд солбилцдог интегратив
- 23. HBV Серотип • SHBsAg (120-163) ← Эсрэгбие • Геномын DR1, DR2, ORF, зарим хэсгийн дараалалд мутаци үүссэн байдаг ба – “а” ерөнхий детеминант бүлэг • “d” субдетерминант + q, x, g • “y” субдетерминант • “r” субдетерминант • “w” субдетерминант /2, 3, 4/
- 25. HBV халдвар нь: • ХИ (элэг халдварлуулах)= 10^10 IU/ml • Тээгчид- 10^6 (1мл-т HBsAg) →↑ • Балархай 10^2 (1мл-т HBsAg) /хуучгүй/ • Биеийн шингэн дэхь HBV түвшин: – Өндөр: цус, ийлдэс, шархны шүүдэс – Дунд: үрийн шингэн, үтрээний шингэн, шүлс – Бага: шээс, баас, хөлс, сүү, нулимс
- 26. Халдварын явц • Цочмог халдвар: 10^6 /1мл/ + ш/т • Архаг халдвар: HBsAg 6 сар ба түүнээс дээш тодорхойлогдох
- 29. HBV output
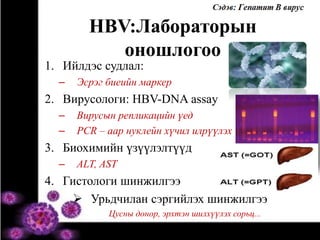
- 30. HBV:Лабораторын оношлогоо 1. Ийлдэс судлал: – Эсрэг биеийн маркер 2. Вирусологи: HBV-DNA assay – Вирусын репликацийн үед – PCR – аар нуклейн хүчил илрүүлэх 3. Биохимийн үзүүлэлтүүд – ALT, AST 4. Гистологи шинжилгээ  Урьдчилан сэргийлэх шинжилгээ Цусны донор, эрхтэн шилхүүлэх сорьц...
- 31. Урьдчилан сэргийлэлт • Идэвхгүй: HBV- иммуноглобулин – Осол/ HBsAg, HBeAg + эхээс төрсөн нярайд 12-48 цагийн дотор • Вакцин (sHBs уураг)– Anti HBs – 1980 онд гаргаж авсан. – 0,1, 6 сар –т 5мг – Дархлаа суларсан 40мг • Дархлаа тогтохгүй. (SHBs-G145R) escape mutation • Нас өндөр, таргалалттай, архинд донтох
- 32. HBV эмчилгээ • Adefovir dipivoxil (Hepsera) • Lamivudine (Epivir HBV) • Interferon alfa (Intron A) – Заалтаар, удаан хугацаатай орших – pgRNA inhibitor – cccDNA inhibitor
- 33. –ì–µ–ø–∞—Ç–∏—Ç—ã–Ω –î –≤–∏—Ä—É—Å Hepatitis D virus / HDV –í–∏—Ä—É—Å–Ω—ã–π –≥–µ–ø–∞—Ç–∏—Ç D
- 34. HDV бүтэц Вирусойдын бүлэг 32-36нм диаметр Бөмбөлөг хэлбэртэй - ssRNA цагираг 1700nt Генотипийн 8 төрөл бүртгэгдсэн
- 35. MP-HDV SHBeAg MHBsAg LHBsAg HBV – н бүрхүүлийг антигентэй нь хамт хуулбарлаж бүрхүүлждэг Ко & Супер халдвар HDV бүтэц SHD – 24kD LHD – 27kD
- 37. HDV халдварын эх уурхай, халдвар дамжих зам Халдварын эх уурхай цочмог болон архаг Д гепатиттай өвчтөн Д гепатит вирус тээгч Халдвар дамжих зам парентераль (цус, бохир багаж, зүү тариур) бэлгийн зам эхээс урагт (вертикаль) Нууц хугацаа 2-6 долоон хоног
- 39. HDV оношилгоо PCR – геном Маркер – Anti-HDV Anti-HDV-IgM Ко-халдвар Супер-халдвар aHBc-IgM + - aHDV-IgM + +
- 40. How E.L.I.S.A.s work –ê–£–°-324 –ì.–ñ–∞—Å—Ä–∞–π
- 41. What is an ELISA? •ELISA = enzyme-linked immunosorbent assay • Enzyme-Linked: enzyme amplifies antigen-antibody interaction • Immunosorbent: reaction products adsorbed on container • Assay: assessment of potential analyte
- 42.  HISTORY  Prior to the development of the EIA/ELISA, the only option for conducting an immunoassay was radioimmunoassay, a technique using radioactively-labeled antigens or antibodies.  Avramais (1966, 1969) and Pierce (1967) developed methods to chemically link antibodies to biological enzymes whose activities produce a measurable signal with solutions containing appropriate substrates.  This signal has to be associated with the presence of antibody or antigen .  COMPONENTS OF ELISA  Antibody: IgG fraction of serum.  Enzyme: Horse Radish Peroxidase (HRP) MW 44, 000, glycoprotein with 4 lysine residues.  Substrate: TMB (3,3',5,5', tetramethylbenzidine) The enzyme acts as a catalyst to oxidize substrate in the presence of Hydrogen peroxide to produce a blue color. Reaction stopped with dilute acid to cause complex to turn yellow.
- 43.  INTRODUCTION TO ELISA A 96 - well microtiter plate being used for ELISA.  A test that uses antibodies and color change to identify a substance.  ELISA is a popular format of a "wet-lab" type analytic biochemistry assay.  ELISA involves at least one antibody with specificity for a particular antigen.  ELISA can perform other forms of ligand binding assays instead of strictly "immuno" assays.
- 44.  PRINCIPLE  “Wet lab" analytic biochemistry assay, ELISA involves detection of an "analyte" in a liquid sample by a method that continues to use liquid reagents during the "analysis“.  The basic principle of an ELISA is to use an enzyme to detect the Ag- Ab binding (antigen- antibody binding). The enzyme converts a colorless substrate (chromogen) to a colored product, indicating the presence of Ag:Ab binding.
- 45. substrate Colored product Secondary antibody Different antigen in sample Primary antibody
- 46.  TYPES OF ELISA  INDIRECT ELISA  DIRECT ELISA  SANDWICH ELISA  COMPETETIVE ELISA NON -COMPETETIVE ELISA
- 47.  INDIRECT ELISA  Antigen is added to plate.  Added Blocking buffer.  Suitable primary antibody is added.  Secondary antibody- HRPO is then added which recognizes and binds to primary antibody.  TMB substrate is added, is converted to detectable form.  Under standard condition ,the enzyme activity measured is proportional to amount of specific antibody in the original serum.
- 48.  ADVANTAGES OF INDIRECT DETECTION  Wide variety of labeled secondary antibodies are available commercially.  Versatile, since many primary antibodies can be made in one species and the Same labeled secondary antibody can be used for detection.  Immunoreactivity of the primary antibody is not affected by labeling.  Sensitivity is increased because each primary antibody contains several epitopes that can be bound by the labeled secondary antibody, allowing for signal amplification.  DISADVANTAGES OF INDIRECT DETECTION  Cross-reactivity may occur with the secondary antibody, resulting in nonspecific signal.  An extra incubation step is required in the procedure.
- 49.  DIRECT ELISA 1. Apply a sample of known antigen to a surface. 2. Enzyme linked primary antibody is applied to the plate. 3. Washed, After this wash, only the antibody-antigen complexes remain attached. 4. Apply a substrate which is converted by the enzyme to elicit a chromogenic signal.  Under standard condition ,the enzyme activity measured is proportional to amount of specific antibody in the original serum.
- 50.  ADVANTAGES OF DIRECT DETECTION  Quick methodology since only one antibody is used.  Cross-reactivity of secondary antibody is eliminated.  DISADVANTAGES OF DIRECT DETECTION  Immunoreactivity of the primary antibody may be reduced as a result of labeling.  Labeling of every primary antibody is time-consuming and expensive.  No flexibility in choice of primary antibody label from one experiment to another.  Little signal amplification.
- 51.  SANDWICH ELISA 1. a. Plate is coated with suitable antibody. b. Blocking buffer is added. 2. Sample is added to plate so antigen is bounded by capture antibody. 3. A suitable biotin labeled detection antibody is added to plate. 4. Enzyme HRPO is added and binds the biotin labeled detection antibody. 5. TMB substrate is added and converted by HRPO to colored product. Under standard condition ,the enzyme activity measured is proportional to the Amount of specific antigen in the original serum.
- 52.  MATERIALS NEEDED FOR ELISA KIT  ELISA Plate  Positive control  Negative control  Dilution Buffer  Conjugate  TMB Substrate  Stop Solution
- 53.  PROCEDURE OF ELISA A B C D E Wash 3X Wash 4X Wash 4X Wash 4X Alkaline phosphatase substrate is added and developed colour is read at 405 nm wavelength to measure plasma cencentration Wells are coated with 0.2 μg primary antibody Diluted plasma is added to coated wells, which bind to antibodies 0.1 μg of biotinylated (biotin = – ) antihuman secondary antibody Incubated overnight at 4˚C Add 1.2000 dilution of streptavidin conjugate to alkaline phosphatase ( E) 2h 2h 1h Incubated at room temperature (24˚C)
- 54.  FINAL PLATE OF ELISA
- 55. COMPETETIVE ELISA  COMPETETIVE ELISA Solid phase coated with antibody Add unknown amount of unlabeled antigen and known amount of labeled antigen Free and labeled antigen are captured Color formation by oxidation of substrate into a colored compound Under standard condition ,the enzyme activity measured is proportional to the proportion of labeled antigen in the mixture of labeled and unlabled antigen.
- 56.  ADVANTAGES:  Suitable for complex (crude or impure) samples, since the antigen does not require purification prior to measurement.  DISADVANTAGES  Each antigen may require a different method to couple it to the enzyme.
- 57.  COMPARISON BETWEEN VARIOUS TYPES OF ELISA Direct ELISA Indirect ELISA Sandwich ELISA Competitive ELISA
- 58.  ELISA RESULTS The ELISA assay yields three different types of data output:  Quantitative: ELISA data can be interpreted in comparison to a standard curve in order to precisely calculate the concentrations of antigen in various samples.  Qualitative: ELISAs can also be used to achieve a yes or no answer indicating whether a particular antigen is present in a sample, as compared to a blank well containing no antigen or an unrelated control antigen.  Semi-quantitative: ELISAs can be used to compare the relative levels of antigen in assay samples, since the intensity of signal will vary directly with antigen concentration.
- 59. ÔÇß ELISA data is typically graphed with Optical density Vs Log concentration to produce a sigmoidal curve. ÔÇß Known concentrations of antigen are used to produce a standard curve and then this data is used to measure the concentration of unknown samples by comparison to the linear portion of the standard curve. ÔÇß This can be done directly on the graph or with curve fitting software which is typically found on ELISA plate readers.
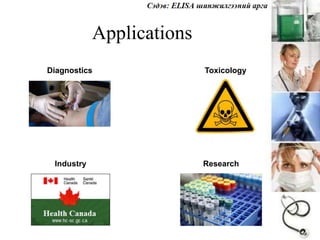
- 60. Applications Diagnostics Toxicology Industry Research
- 61.  APPLICATIONS  Screening donated blood for evidence of viral contamination by  HIV-1 and HIV-2 (presence of anti-HIV antibodies)  Hepatitis C (presence of antibodies)  Hepatitis B (testing for both antibodies and a viral antigen)  Measuring hormone levels  HCG (as a test for pregnancy)  LH (determining the time of ovulation)  TSH, T3 and T4 (for thyroid function)  Detecting infections  Sexually-transmitted agents like HIV, syphilis and chlamydia  Hepatitis B and C  Toxoplasma gondii , H.pylori  Detecting illicit drugs.  Detecting allergens in food and house dust
- 62. Diagnostic Applications (Home) •Pregnancy test • hCG hormone in pregnant woman’s urine • 1-10 min: (+) two lines, (-) one line HIV test • Oral fluid: OraSure, OraQuick, Calypte AWARE • Blood: Home Access
- 63. Industrial Applications •Allergen detection • 8 major food allergens • Detection at low levels (ppm) • Alerts for contamination •Vaccine quality control • Licensing • Validate consistency of production
- 64. Toxicological applications •Prescription medication • Cardiovascular drugs, antidepressants, chemotherapeutics • Drug dosing •Drugs of abuse • Morphine, amphetamines, barbiturates • Newborns
- 65.  SENSITIVITY ELISAs are one of the most sensitive immunoassays available. The typical detection range for an ELISA is 0.1 to 1 fmole or 0.01 ng to 0.1 ng, with sensitivity dependent upon the particular characteristics of the antibody – antigen interaction. In addition, some substrates such as those yielding enhanced chemiluminescent or fluorescent signal, can be used to improve results. As mentioned earlier, indirect detection will produce higher levels of signal and should therefore be more sensitive. However, it can also cause higher background signal thus reducing net specific signal levels.
- 66. Sensitivity and Specificity •Sensitivity • Probability: positive test for a patient with disease • Takes false negatives into account • More important for ELISA •Specificity • Probability: negative test for a patient without disease • Takes false positives into account •ELISA has high sensitivity (>99%) and high specificity (>99%) • Not perfect: false positives/negatives can occur
Editor's Notes
- #61: Picture 1: http://diet-solution.net/5-reasons-for-having-your-blood-tested/ Picture 2: http://www.goodnewsfinland.com/archive/themes/diagnostics/diagnostics-generates-growth/ Picture 3: http://caep.ca/cpdcme/roadshows-current-cme/toxicology Picture 4: http://global.unc.edu/news/unc-toxicologists-present-nexgen-tools-to-canadas-national-health-agency/
- #63: Chard, T. (1992). Review: pregnancy tests: a review. Human Reproduction, 7(5), 701-710. Dipiro, J.T., Talbert, R.L., Yee, G.C., Matzke, G.R., Wells, B.G., & Posey, L.M. (2008): Pharmacotherapy A Pathophysiologic Approach Seventh Edition. New York: McGraw-Hill Companies Inc. Picture 1: http://newdadtobe.com/post-2/ Picture 2: http://www.oraquick.com/Taking-the-Test/Understanding-Your-Results
- #64: Le, C. T., Grambsch, P. M., & Giebink, G.S. (2003). Quality control and the identification of vaccine responders using ELISA-derived antibody data. Statistics in Medicine, 22(18), 2935-2942. Stephan, O., & Vieths, S. (2004). Development of a real-time PCR and a sandwich ELISA for detection of potentially allergenic trace amounts of peanut (Arachis hypogaea) in processed foods. Journal of Agricultural and Food Chemistry, 52(12), 3754-3760. Picture 1: http://www.ifood.tv/blog/nut-free-snacks Picture 2: http://www.wisegeek.com/what-is-needle-phobia.htm
- #65: Picture 1: http://www.simmlands.com/wp-content/uploads/2011/05/gavel-and-book-300x199.jpg
- #67: Dipiro, J.T., Talbert, R.L., Yee, G.C., Matzke, G.R., Wells, B.G., & Posey, L.M. (2008): Pharmacotherapy A Pathophysiologic Approach Seventh Edition. New York: McGraw-Hill Companies Inc.