PROPERTIES-OF-MATTER chemistry lesson q1
Download as PPTX, PDF0 likes5 views
chemistry
1 of 44
Download to read offline











































Recommended
Matter and materials



Matter and materialsMxolisi Creswell MNCUBE
╠²
1. The document discusses various properties of matter and how they are used to classify and identify different types of matter. It describes extensive properties that depend on amount and intensive properties that depend on type.
2. Mixtures and pure substances are introduced. Heterogeneous mixtures are non-uniform while homogeneous mixtures are uniform throughout. Elements have a unique set of properties while compounds contain two or more elements.
3. The three states of matter are defined as solid, liquid, and gas. Physical and chemical changes are distinguished based on whether the composition changes. Chemical symbols and formulas are used to represent elements and compounds in chemical reactions.Science chapter 2!



Science chapter 2!lyndseyb
╠²
This document discusses matter and its properties. It defines matter as either pure substances or mixtures. Pure substances are either elements or compounds, while mixtures can be homogeneous or heterogeneous. The document then discusses several physical properties of matter like viscosity, conductivity, malleability, hardness, density, melting point, and boiling point. It explains processes like distillation, filtration, evaporation, and electrolysis that are used to separate mixtures based on these physical properties. The review questions ask about examples of physical changes, classifying mixtures, why mixtures vary, and separation processes.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundstracyconover
╠²
Mixtures and pure substances can be categorized and separated in different ways. Mixtures are combinations of substances that are not chemically combined and can be physically separated. Pure substances include elements, which consist of only one type of atom, and compounds, which are formed by chemical combination of two or more elements. Mixtures include heterogeneous mixtures where the parts can be easily distinguished, like mixtures, and homogeneous mixtures where the parts are evenly distributed and appear uniform, like solutions.Ch 2 materials



Ch 2 materialskeelanwgraland
╠²
The document defines key chemistry concepts such as elements, compounds, mixtures, solutions, suspensions, colloids, and separation techniques including distillation, evaporation, and filtration. It also discusses chemical changes and provides examples such as changes in color, temperature, production of gas, or formation of precipitate. Physical and chemical changes are compared. Reactivity and flammability are also addressed. Review questions are included to test understanding.Ch 2 materials



Ch 2 materialskeelanwgraland
╠²
The document defines key chemistry concepts such as elements, compounds, mixtures, solutions, suspensions, colloids, and separation techniques including distillation, evaporation, and filtration. It also discusses chemical changes and provides examples such as changes in color, temperature, production of gas, or formation of precipitate. Physical and chemical changes are compared. Reactivity and flammability are also addressed. Review questions are included to test understanding.Properties of matter pp



Properties of matter ppmariafpassarelli
╠²
This document discusses the properties of matter including pure substances like elements and compounds, as well as mixtures. It describes the differences between heterogeneous and homogeneous mixtures and how mixtures can be classified based on particle size. The document also covers physical properties, chemical properties, and how physical and chemical changes can be distinguished.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundstracyconover
╠²
Mixtures and pure substances can be categorized and separated using various physical properties. Mixtures are combinations of substances that are not chemically combined and can be separated through processes like distillation or filtration. Solutions are homogeneous mixtures that appear and distribute uniformly. Suspensions and colloids are heterogeneous mixtures where particles settle or scatter light differently. Elements are pure substances made of only one type of atom that cannot be broken down further. Compounds are pure substances made of two or more elements chemically bonded together with unique properties.Elements compounds and mixtures notes



Elements compounds and mixtures notesknewton1314
╠²
This document discusses the differences between elements, compounds, and mixtures. It defines each term and provides examples. Mixtures are combinations of substances that are not chemically combined and can be separated through physical means. Solutions, suspensions, and colloids are types of mixtures. Elements are pure substances that cannot be broken down further, while compounds are pure substances composed of two or more chemically bonded elements.Matter



Matteratreasuredsecret
╠²
Chemistry is the study of matter and its properties. Matter can exist as elements, compounds or mixtures. Elements are pure substances that cannot be broken down further. Compounds are formed by chemical bonds between different elements. Mixtures maintain their individual chemical properties and can be separated physically. The kinetic molecular theory describes matter at the molecular level in terms of motion and energy. A physical change alters a substance's physical properties without changing its chemical makeup, while a chemical change produces new substances.Sarah's Science Review Powerpoint



Sarah's Science Review PowerpointSarahMerrifield
╠²
Density is a physical property that is defined as mass divided by volume. It can be used to compare unknown solids by measuring their densities. Density can be measured using a balance to find mass and a ruler or graduated cylinder to find volume. A suspension is a type of mixture that is distinguished by having distinct layers and particles that can be seen settling. A mixture is a physical combination of substances that can be separated physically, while a compound is a chemical combination that can only be separated chemically and exists in fixed ratios with unique properties.Science party



Science partyNathanRosenberg
╠²
There are currently 118 known elements that make up all matter. Elements are pure substances that contain only one type of atom, while compounds are made of two or more elements or other compounds. Mixtures have a variable composition because their ingredients are not uniformly distributed.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundsjdrin001
╠²
This document defines and provides examples of mixtures, solutions, suspensions, and colloids. It explains that mixtures can be physically separated into their original substances, while solutions appear homogeneous but are mixtures of a solute dissolved in a solvent on a molecular level. Suspensions and colloids are heterogeneous mixtures where particles settle out or scatter light differently. The document also defines elements as pure substances that cannot be broken down further, and compounds as pure substances formed by a chemical combination of elements in fixed ratios that have properties distinct from the original elements.IS 4th Quarter



IS 4th QuarterAronn Angelo Noel
╠²
The document provides definitions and descriptions of key chemistry concepts including the different states of matter, properties of substances, classification of pure substances and mixtures, and examples of common chemical reactions and separation techniques. Key topics covered include metals, non-metals, acids, bases, solutions, and heterogeneous and homogeneous mixtures.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundsAllyse Fritz
╠²
This document defines and provides examples of mixtures, solutions, suspensions, colloids, and gas mixtures. It explains that mixtures can be separated physically but their compositions are not fixed, while solutions appear homogeneous but are mixtures that dissolve. Suspensions and colloids are mixtures where particles settle or scatter light differently. The document also defines elements as pure substances that cannot be broken down, and compounds as pure substances formed by chemical combination of elements in specific ratios producing new properties.Made by aman



Made by amanAshish Phogat
╠²
This document discusses key concepts in chemistry including:
- Chemistry is the study of matter which is anything that has mass and takes up space. All matter is made of atoms.
- Pure substances can be elements, which contain only one type of atom, or compounds, which contain two or more elements in a fixed ratio. Mixtures contain two or more substances mixed together.
- Properties describe characteristics of matter and can be physical, relating to a substance's observable properties, or chemical, relating to how it interacts with other substances.
- A chemical change forms new substances while a physical change only alters a substance's physical properties like state.
- Chemical tests can identify substances by their reactions and common tests are describedBasics of Matter.ppt



Basics of Matter.pptGraceAceveda
╠²
This document discusses physical and chemical changes, pure substances, mixtures, and states of matter. It defines physical changes as changes in a substance's state or form without changing its chemical composition, and chemical changes as the formation of new substances through chemical reactions. Mixtures are combinations of substances that are not chemically bonded and can be separated by physical means. The four common states of matter are solids, liquids, gases, and plasma, which differ in the arrangement and movement of their particles according to the kinetic molecular theory.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundstracyconover
╠²
This document defines and provides examples of mixtures, solutions, suspensions, colloids, and gas mixtures. It explains that mixtures can be physically separated into their original substances, while solutions appear homogeneous. Suspensions can settle out but colloids cannot. It also defines elements as pure substances that cannot be broken down further, and compounds as pure substances formed by a chemical combination of elements in fixed ratios that have unique properties.Keynote!!



Keynote!!emmacgraland
╠²
This document defines and describes various chemistry concepts including:
1. Distillation is a process that separates substances in a solution based on their boiling points. Evaporation is the process where a liquid becomes a gas.
2. Evidence of a chemical reaction includes a change in color, formation of a gas, or formation of a precipitate.
3. Mixtures contain two or more substances that are not chemically combined and can be separated by physical means, while elements and compounds have fixed compositions.Classifications of Matter



Classifications of MatterSimple ABbieC
╠²
This document defines the classification of matter. There are two main categories: pure substances and mixtures. Pure substances include elements, which are made of only one type of atom, and compounds, which are two or more elements chemically bonded together. Mixtures contain two or more pure substances mixed together without chemical bonding. Mixtures can be either heterogeneous, where the parts can be seen, or homogeneous, where the parts cannot be seen. Heterogeneous mixtures are less pure than homogeneous mixtures.Chapter 1 typed.pptx



Chapter 1 typed.pptxMegan968040
╠²
This document provides an introduction to general chemistry, including why chemistry is studied, its central role in understanding matter, and learning goals for the course. Chemistry involves understanding the properties and behavior of matter, which exists as elements, compounds, and mixtures. The three states of matter - solid, liquid, and gas - are classified based on molecular motion and energy. Physical and chemical properties help characterize different types of pure substances and mixtures. Changes in matter can involve physical changes of state or chemical reactions that alter chemical identity. Energy also plays a key role in these transformations.Text slideshow



Text slideshowEliasGiron
╠²
This document defines key chemistry concepts such as elements, compounds, mixtures, and the different types of mixtures. It discusses homogeneous mixtures like solutions, and heterogeneous mixtures like colloids. It also covers physical properties including viscosity, conductivity, malleability, melting and boiling points. Finally, it discusses processes such as filtration, distillation, evaporation, and the differences between chemical and physical changes.Substances and Mixtures



Substances and MixturesMelinda MacDonald
╠²
This document discusses the differences between substances, mixtures, and compounds. It defines a substance as matter made of the same atoms, and notes that elements and compounds are types of substances. A mixture is two or more substances physically blended but not chemically bonded. Granite and air are given as examples of heterogeneous and homogeneous mixtures respectively. The document explains that mixtures can be separated into their original substances using physical means, while compounds require chemical changes to separate them into their component elements.Matter



Matterpalmanojav
╠²
The document discusses the different states and properties of matter. It defines matter as anything that has mass and occupies space, and identifies the three normal states as solid, liquid, and gas. At extremely high or low temperatures, plasma and Bose-Einstein condensate exist as the 4th and 5th states. Matter has physical properties like mass, volume, and extensive/intensive properties, as well as chemical properties regarding its composition and ability to undergo chemical reactions. Physical changes alter the state of matter without changing composition, while chemical changes result in new substances through reactions. Examples of corrosion show unwanted oxidation of metals.Introduction to Chemistry Gr. 7 2018 



Introduction to Chemistry Gr. 7 2018 Ruba Salah
╠²
This document introduces elements, compounds, and mixtures. It defines an element as a pure substance made of only one type of atom. Compounds are composed of two or more elements that are chemically bonded together into molecules. Compounds have different properties than their constituent elements. Mixtures are combinations of substances that are not chemically bonded and can be separated through physical means unlike compounds. The document provides examples of elements and properties of metals and non-metals to classify elements and distinguish compounds from mixtures.Matter physical chemical properties



Matter physical chemical propertiesBridget.Bradshaw
╠²
This document discusses physical and chemical properties and changes of matter. It defines matter as anything that has mass and occupies space, while energy has no mass and occupies no space. Matter is composed of atoms, which are made up of protons, neutrons, and electrons. Pure substances like elements and compounds have a constant composition, while mixtures can have variable compositions and can be either homogeneous or heterogeneous. Physical changes do not alter the chemical makeup of a substance, and involve changes in state, shape, or size. Chemical changes result in new substances forming through reactions that alter chemical compositions. Chemical properties involve reactions, while physical properties can be observed without reactions occurring.Chapter 2 Powerpoint



Chapter 2 Powerpointclairewgraland
╠²
This document discusses the properties of matter and different types of mixtures and changes. It defines elements as substances with only one type of atom, and compounds as two or more elements joined in fixed proportions. Mixtures can have varying compositions. A suspension is a mixture where the largest particles are visible. Filtration and distillation are common separation methods that separate based on particle size and boiling point. Physical changes do not alter composition while chemical changes produce a new substance.Matter and its properties



Matter and its propertiesChris Mack
╠²
This document discusses the fundamental properties and classification of matter. It defines matter as anything that has mass and takes up space, and it is made of atoms which combine to form elements or compounds. The properties of matter can be extensive, depending on amount, or intensive, not depending on amount. Matter exists in solid, liquid, gas and plasma states and undergoes physical changes that do not alter its chemical identity or chemical changes that create new substances. Mixtures are combinations of substances that retain their own properties, while pure substances have consistent composition and properties regardless of sample.TOP 10 CBSE Top Science Projects for Classes 6 to 10 with Youtube Tutorial



TOP 10 CBSE Top Science Projects for Classes 6 to 10 with Youtube TutorialVivek Bhakta
╠²
Top 10 CBSE Science Projects for Classes 6 to 10 | Easy DIY Models with YouTube Tutorial
Looking for the best CBSE science projects for Classes 6 to 10? HereŌĆÖs a collection of Top 10 working models that are perfect for science exhibitions, school projects, and STEM learning. These projects cover essential science concepts from physics, chemistry, and biology, making them both fun and educational.
Each project includes a step-by-step YouTube tutorial, so students can easily follow along and build their own models.
Top 10 CBSE Science Projects for Classes 6 to 10:
1’ĖÅŌāŻ Hydraulic Bridge Model ŌĆō Demonstrate the principles of hydraulics and PascalŌĆÖs Law.
2’ĖÅŌāŻ Electric Motor Model ŌĆō Understand how electromagnetism powers motors.
3’ĖÅŌāŻ Solar-Powered Car ŌĆō Explore renewable energy and motion mechanics.
4’ĖÅŌāŻ Wind Turbine Generator ŌĆō Convert wind energy into electrical power.
5’ĖÅŌāŻ Automatic Street Light System ŌĆō Learn about LDR sensors and energy efficiency.
6’ĖÅŌāŻ Water Dispenser Model ŌĆō Show the role of air pressure in fluid movement.
7’ĖÅŌāŻ Earthquake Alarm System ŌĆō Build a vibration-based alert system for disaster safety.
8’ĖÅŌāŻ Biogas Plant Model ŌĆō Explain how organic waste is converted into energy.
9’ĖÅŌāŻ Rainwater Harvesting Model ŌĆō Demonstrate sustainable water conservation techniques.
¤ö¤ Smart Irrigation System ŌĆō Create an automated plant watering system using sensors.
Why Choose These Projects?
Ō£ö Simple & Fun ŌĆō Uses easily available materials.
Ō£ö Educational & Practical ŌĆō Covers key CBSE science topics.
Ō£ö YouTube Video Guide ŌĆō Step-by-step tutorials for easy learning.
¤öŚ Watch the full YouTube tutorial and start building your project today! More Related Content
Similar to PROPERTIES-OF-MATTER chemistry lesson q1 (20)
Matter



Matteratreasuredsecret
╠²
Chemistry is the study of matter and its properties. Matter can exist as elements, compounds or mixtures. Elements are pure substances that cannot be broken down further. Compounds are formed by chemical bonds between different elements. Mixtures maintain their individual chemical properties and can be separated physically. The kinetic molecular theory describes matter at the molecular level in terms of motion and energy. A physical change alters a substance's physical properties without changing its chemical makeup, while a chemical change produces new substances.Sarah's Science Review Powerpoint



Sarah's Science Review PowerpointSarahMerrifield
╠²
Density is a physical property that is defined as mass divided by volume. It can be used to compare unknown solids by measuring their densities. Density can be measured using a balance to find mass and a ruler or graduated cylinder to find volume. A suspension is a type of mixture that is distinguished by having distinct layers and particles that can be seen settling. A mixture is a physical combination of substances that can be separated physically, while a compound is a chemical combination that can only be separated chemically and exists in fixed ratios with unique properties.Science party



Science partyNathanRosenberg
╠²
There are currently 118 known elements that make up all matter. Elements are pure substances that contain only one type of atom, while compounds are made of two or more elements or other compounds. Mixtures have a variable composition because their ingredients are not uniformly distributed.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundsjdrin001
╠²
This document defines and provides examples of mixtures, solutions, suspensions, and colloids. It explains that mixtures can be physically separated into their original substances, while solutions appear homogeneous but are mixtures of a solute dissolved in a solvent on a molecular level. Suspensions and colloids are heterogeneous mixtures where particles settle out or scatter light differently. The document also defines elements as pure substances that cannot be broken down further, and compounds as pure substances formed by a chemical combination of elements in fixed ratios that have properties distinct from the original elements.IS 4th Quarter



IS 4th QuarterAronn Angelo Noel
╠²
The document provides definitions and descriptions of key chemistry concepts including the different states of matter, properties of substances, classification of pure substances and mixtures, and examples of common chemical reactions and separation techniques. Key topics covered include metals, non-metals, acids, bases, solutions, and heterogeneous and homogeneous mixtures.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundsAllyse Fritz
╠²
This document defines and provides examples of mixtures, solutions, suspensions, colloids, and gas mixtures. It explains that mixtures can be separated physically but their compositions are not fixed, while solutions appear homogeneous but are mixtures that dissolve. Suspensions and colloids are mixtures where particles settle or scatter light differently. The document also defines elements as pure substances that cannot be broken down, and compounds as pure substances formed by chemical combination of elements in specific ratios producing new properties.Made by aman



Made by amanAshish Phogat
╠²
This document discusses key concepts in chemistry including:
- Chemistry is the study of matter which is anything that has mass and takes up space. All matter is made of atoms.
- Pure substances can be elements, which contain only one type of atom, or compounds, which contain two or more elements in a fixed ratio. Mixtures contain two or more substances mixed together.
- Properties describe characteristics of matter and can be physical, relating to a substance's observable properties, or chemical, relating to how it interacts with other substances.
- A chemical change forms new substances while a physical change only alters a substance's physical properties like state.
- Chemical tests can identify substances by their reactions and common tests are describedBasics of Matter.ppt



Basics of Matter.pptGraceAceveda
╠²
This document discusses physical and chemical changes, pure substances, mixtures, and states of matter. It defines physical changes as changes in a substance's state or form without changing its chemical composition, and chemical changes as the formation of new substances through chemical reactions. Mixtures are combinations of substances that are not chemically bonded and can be separated by physical means. The four common states of matter are solids, liquids, gases, and plasma, which differ in the arrangement and movement of their particles according to the kinetic molecular theory.Mixtures, solutions, elements, compounds



Mixtures, solutions, elements, compoundstracyconover
╠²
This document defines and provides examples of mixtures, solutions, suspensions, colloids, and gas mixtures. It explains that mixtures can be physically separated into their original substances, while solutions appear homogeneous. Suspensions can settle out but colloids cannot. It also defines elements as pure substances that cannot be broken down further, and compounds as pure substances formed by a chemical combination of elements in fixed ratios that have unique properties.Keynote!!



Keynote!!emmacgraland
╠²
This document defines and describes various chemistry concepts including:
1. Distillation is a process that separates substances in a solution based on their boiling points. Evaporation is the process where a liquid becomes a gas.
2. Evidence of a chemical reaction includes a change in color, formation of a gas, or formation of a precipitate.
3. Mixtures contain two or more substances that are not chemically combined and can be separated by physical means, while elements and compounds have fixed compositions.Classifications of Matter



Classifications of MatterSimple ABbieC
╠²
This document defines the classification of matter. There are two main categories: pure substances and mixtures. Pure substances include elements, which are made of only one type of atom, and compounds, which are two or more elements chemically bonded together. Mixtures contain two or more pure substances mixed together without chemical bonding. Mixtures can be either heterogeneous, where the parts can be seen, or homogeneous, where the parts cannot be seen. Heterogeneous mixtures are less pure than homogeneous mixtures.Chapter 1 typed.pptx



Chapter 1 typed.pptxMegan968040
╠²
This document provides an introduction to general chemistry, including why chemistry is studied, its central role in understanding matter, and learning goals for the course. Chemistry involves understanding the properties and behavior of matter, which exists as elements, compounds, and mixtures. The three states of matter - solid, liquid, and gas - are classified based on molecular motion and energy. Physical and chemical properties help characterize different types of pure substances and mixtures. Changes in matter can involve physical changes of state or chemical reactions that alter chemical identity. Energy also plays a key role in these transformations.Text slideshow



Text slideshowEliasGiron
╠²
This document defines key chemistry concepts such as elements, compounds, mixtures, and the different types of mixtures. It discusses homogeneous mixtures like solutions, and heterogeneous mixtures like colloids. It also covers physical properties including viscosity, conductivity, malleability, melting and boiling points. Finally, it discusses processes such as filtration, distillation, evaporation, and the differences between chemical and physical changes.Substances and Mixtures



Substances and MixturesMelinda MacDonald
╠²
This document discusses the differences between substances, mixtures, and compounds. It defines a substance as matter made of the same atoms, and notes that elements and compounds are types of substances. A mixture is two or more substances physically blended but not chemically bonded. Granite and air are given as examples of heterogeneous and homogeneous mixtures respectively. The document explains that mixtures can be separated into their original substances using physical means, while compounds require chemical changes to separate them into their component elements.Matter



Matterpalmanojav
╠²
The document discusses the different states and properties of matter. It defines matter as anything that has mass and occupies space, and identifies the three normal states as solid, liquid, and gas. At extremely high or low temperatures, plasma and Bose-Einstein condensate exist as the 4th and 5th states. Matter has physical properties like mass, volume, and extensive/intensive properties, as well as chemical properties regarding its composition and ability to undergo chemical reactions. Physical changes alter the state of matter without changing composition, while chemical changes result in new substances through reactions. Examples of corrosion show unwanted oxidation of metals.Introduction to Chemistry Gr. 7 2018 



Introduction to Chemistry Gr. 7 2018 Ruba Salah
╠²
This document introduces elements, compounds, and mixtures. It defines an element as a pure substance made of only one type of atom. Compounds are composed of two or more elements that are chemically bonded together into molecules. Compounds have different properties than their constituent elements. Mixtures are combinations of substances that are not chemically bonded and can be separated through physical means unlike compounds. The document provides examples of elements and properties of metals and non-metals to classify elements and distinguish compounds from mixtures.Matter physical chemical properties



Matter physical chemical propertiesBridget.Bradshaw
╠²
This document discusses physical and chemical properties and changes of matter. It defines matter as anything that has mass and occupies space, while energy has no mass and occupies no space. Matter is composed of atoms, which are made up of protons, neutrons, and electrons. Pure substances like elements and compounds have a constant composition, while mixtures can have variable compositions and can be either homogeneous or heterogeneous. Physical changes do not alter the chemical makeup of a substance, and involve changes in state, shape, or size. Chemical changes result in new substances forming through reactions that alter chemical compositions. Chemical properties involve reactions, while physical properties can be observed without reactions occurring.Chapter 2 Powerpoint



Chapter 2 Powerpointclairewgraland
╠²
This document discusses the properties of matter and different types of mixtures and changes. It defines elements as substances with only one type of atom, and compounds as two or more elements joined in fixed proportions. Mixtures can have varying compositions. A suspension is a mixture where the largest particles are visible. Filtration and distillation are common separation methods that separate based on particle size and boiling point. Physical changes do not alter composition while chemical changes produce a new substance.Matter and its properties



Matter and its propertiesChris Mack
╠²
This document discusses the fundamental properties and classification of matter. It defines matter as anything that has mass and takes up space, and it is made of atoms which combine to form elements or compounds. The properties of matter can be extensive, depending on amount, or intensive, not depending on amount. Matter exists in solid, liquid, gas and plasma states and undergoes physical changes that do not alter its chemical identity or chemical changes that create new substances. Mixtures are combinations of substances that retain their own properties, while pure substances have consistent composition and properties regardless of sample.Recently uploaded (20)
TOP 10 CBSE Top Science Projects for Classes 6 to 10 with Youtube Tutorial



TOP 10 CBSE Top Science Projects for Classes 6 to 10 with Youtube TutorialVivek Bhakta
╠²
Top 10 CBSE Science Projects for Classes 6 to 10 | Easy DIY Models with YouTube Tutorial
Looking for the best CBSE science projects for Classes 6 to 10? HereŌĆÖs a collection of Top 10 working models that are perfect for science exhibitions, school projects, and STEM learning. These projects cover essential science concepts from physics, chemistry, and biology, making them both fun and educational.
Each project includes a step-by-step YouTube tutorial, so students can easily follow along and build their own models.
Top 10 CBSE Science Projects for Classes 6 to 10:
1’ĖÅŌāŻ Hydraulic Bridge Model ŌĆō Demonstrate the principles of hydraulics and PascalŌĆÖs Law.
2’ĖÅŌāŻ Electric Motor Model ŌĆō Understand how electromagnetism powers motors.
3’ĖÅŌāŻ Solar-Powered Car ŌĆō Explore renewable energy and motion mechanics.
4’ĖÅŌāŻ Wind Turbine Generator ŌĆō Convert wind energy into electrical power.
5’ĖÅŌāŻ Automatic Street Light System ŌĆō Learn about LDR sensors and energy efficiency.
6’ĖÅŌāŻ Water Dispenser Model ŌĆō Show the role of air pressure in fluid movement.
7’ĖÅŌāŻ Earthquake Alarm System ŌĆō Build a vibration-based alert system for disaster safety.
8’ĖÅŌāŻ Biogas Plant Model ŌĆō Explain how organic waste is converted into energy.
9’ĖÅŌāŻ Rainwater Harvesting Model ŌĆō Demonstrate sustainable water conservation techniques.
¤ö¤ Smart Irrigation System ŌĆō Create an automated plant watering system using sensors.
Why Choose These Projects?
Ō£ö Simple & Fun ŌĆō Uses easily available materials.
Ō£ö Educational & Practical ŌĆō Covers key CBSE science topics.
Ō£ö YouTube Video Guide ŌĆō Step-by-step tutorials for easy learning.
¤öŚ Watch the full YouTube tutorial and start building your project today! Automating Compression Ultrasonography of Human Thigh Tissue and Vessels via ...



Automating Compression Ultrasonography of Human Thigh Tissue and Vessels via ...ThrombUS+ Project
╠²
Rytis Jurkonis from Kaunas University of Technology (Lithuania) presented their recent work entitled ŌĆ£Automating Compression Ultrasonography of Human Thigh Tissue and Vessels via Strain Estimation." Rytis presented on the methodology along the novel wearable hardware developed to automate compression ultrasonography for DVT detection in the lower limbs. In addition, preliminary results were shared, highlighting the feasibility of an operator-independent method to perform compression ultrasonography.
Presented at BIOSTEC 2025 in Porto, Portugal.
About ThrombUS+: Our interdisciplinary approach centers around creating a novel wearable diagnostic device utilizing autonomous, AI-driven DVT detection. This groundbreaking device incorporates wearable ultrasound hardware, impedance plethysmography, and light reflection rheography for early clot detection. ThrombUS+ is designed for postoperative patients, those undergoing lengthy surgical procedures, cancer patients, bedridden individuals at home or in care units, and women during pregnancy and postpartum.2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...



2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...Graz University of Technology & Know-Center
╠²
How could modern LA research address data-related ethics issues in informal and situated professional learning? I will identify in this talk three relevant insights based on field studies around workplace LA interventions: Firstly, in informal and situated learning, data isnŌĆÖt just about the learners. Secondly, the affordances of manual and automatic data tracking for learning are very different, with manual tracking allowing a high degree of learner control over data. Thirdly, learning is not necessarily a shared goal in workplaces. These can be translated into seeing a potential for systems endowed with sufficient natural-language-processing capability (now seemingly at our fingertips with LLMs), and socio-technical design and scenario-based data collection analysis as design and research methods.Unjustly Incriminating Bacteria: the Role of Bacteriophages in Bacterial Infe...



Unjustly Incriminating Bacteria: the Role of Bacteriophages in Bacterial Infe...christianagboeze2427
╠²
SUMMARY
Based on human relationship with bacteria, virulence is one of the most important case to us. Some forms of virulence thought to arise only from the actions of bacteria are not actually caused by them but are indirectly influenced by another counterpart in the microbial mix of the ecosystem called bacteriophage; viruses that only infect prokaryotes such as bacteria but not eukaryotes. Bacteriophages preferably attack bacteria due to the lack of specific receptors for phages on eukaryotic cells which are found in bacteria e.g. peptide sequences and polysaccharide moieties in gram positive and gram negative bacteria, bacterial capsules, slime layers, flagella etc. They recognize and bind to bacteria using appropriate receptors, subsequently proceeding to inject their genome called prophage into their host. This review focuses on the most probable outcomes of phage-host interactions via the lytic and lysogenic cycles which are therapeutic effect and pathogenicity/resistance to antibiotics respectively. By lysogenic conversion or transfer of acquired genetic materials via transduction, phages can confer unusual traits such as virulence and antibiotics resistance. Important pathogenic bacteria that cause persistent and critical infections which have their pathogenicity engineered by phages include Pseudomonas aeruginosa, Salmonella enterica, Escherichia coli, Vibrio cholerae, Staphylococcus spp., and Clostridium spp.
The prophages influence their virulence in a variety of ways which include: contribution to the production of phage-encoded toxins, modification of the bacterial envelope, mediation of bacterial infectivity, and control of bacterial cell regulation. The unwavering threat of antimicrobial resistance in global health, extreme difficulty involved in developing novel antibiotics, and the rate at which microorganisms develop resistance to newly introduced antimicrobials have sparked urgency and interest in research for effective methods to eradicate pathogenic bacteria and limit antibiotic resistance. As a result, interest in phage therapy has been reignited because of the high efficiency in detecting and killing pathogenic bacteria by phages.
Units and measurements includes definition and fundamental quantities.pptx



Units and measurements includes definition and fundamental quantities.pptxDr Sarika P Patil
╠²
Some definitions, Physical quantities, fundamental quantity are discussed in PPTepidemiology (aim, component, principles).pptx



epidemiology (aim, component, principles).pptxlopamudraray88
╠²
To study historically the rise and fall of disease in the population.
Community diagnosis.
Planning and evaluation.
Evaluation of individuals risks and chances.
Completing the natural history of disease.
Searching for causes and risk factors.
ARepeatingFastRadioBurstSourceinaLow-luminosityDwarfGalaxy



ARepeatingFastRadioBurstSourceinaLow-luminosityDwarfGalaxyS├®rgio Sacani
╠²
Wepresent the localizationandhostgalaxyofFRB20190208A, arepeatingsourceof fast radiobursts (FRBs) discoveredusingCHIME/FRB.Aspartof thePinpointingREpeatingChImeSourceswithEVNdishesrepeater localizationprogramon theEuropeanVLBINetwork (EVN),wemonitoredFRB20190208Afor 65.6hr at Ōł╝1.4GHzanddetectedasingleburst,whichledtoitsverylongbaselineinterferometrylocalizationwith260mas uncertainty(2Žā).Follow-upopticalobservationswiththeMMTObservatory(i’éē25.7mag(AB))foundnovisible hostattheFRBposition.SubsequentdeeperobservationswiththeGranTelescopioCanarias,however,revealedan extremelyfaintgalaxy(r=27.32┬▒0.16mag),verylikely(99.95%)associatedwithFRB20190208A.Giventhe dispersionmeasureoftheFRB(Ōł╝580pccmŌłÆ3),eventhemostconservativeredshiftestimate( ~ z 0.83 max )implies TheAstrophysicalJournalLetters,977:L4(17pp),2024December10 https://doi.org/10.3847/2041-8213/ad8ce1 ┬®2024.TheAuthor(s).PublishedbytheAmericanAstronomicalSociety. 30BantingFellow. 31McGillSpaceInstituteFellow. 32 FRQNTPostdoctoralFellow. Originalcontent fromthisworkmaybeusedunder theterms of theCreativeCommonsAttribution4.0licence.Anyfurther distributionofthisworkmustmaintainattributiontotheauthor(s)andthetitle of thework, journalcitationandDOI. 1The Astrophysical Journal Letters, 977:L4 (17pp), 2024 December 10 Hewitt et al. that this is the lowest-luminosity FRB host to date (’éł108 Le), even less luminous than the dwarf host of FRB20121102A. We investigate how localization precision and the depth of optical imaging affect host association and discuss the implications of such a low-luminosity dwarf galaxy. Unlike the other repeaters with low-luminosity hosts, FRB 20190208A has a modest Faraday rotation measure of a few tens of rad mŌłÆ2, and EVN plus Very Large Array observations reveal no associated compact persistent radio source. We also monitored FRB20190208A for 40.4hr over 2yr as part of the Extragalactic Coherent Light from Astrophysical Transients repeating FRB monitoring campaign on the Nan├¦ay Radio Telescope and detected one burst. Our results demonstrate that, in some cases, the robust association of an FRB with a host galaxy will require both high localization precision and deep optical follow-up. Unified Astronomy Thesaurus concepts: Radio bursts (1339); Radio transient sources (2008); Very long baseline interferometry (1769); Dwarf galaxies (416)CONDUCTOMETRY presentation for MSc students.pptx



CONDUCTOMETRY presentation for MSc students.pptxNakulBarwat
╠²
Conductometry presentation by our student Successful management of intussusception in a cow under double drip anaesthesia



Successful management of intussusception in a cow under double drip anaesthesiarajvet4163
╠²
Intussusception in a crossbred cow
surgical treatment, double drip anaesthesia and complete recovery of animal with case discussionWORKING AND APPLICATION OF LC-MS/MS 2025



WORKING AND APPLICATION OF LC-MS/MS 2025PSG College of Technology
╠²
LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry) is a powerful analytical tool for comparing innovator and biosimilar drugs. It ensures precise characterization, detecting structural variations, impurities, and post-translational modifications, ensuring biosimilar quality, efficacy, and regulatory compliance in pharmaceutical development.Climate Information for Society: Attribution and Engineering



Climate Information for Society: Attribution and EngineeringZachary Labe
╠²
28-30 January 2025ŌĆ”
OAR GFDL 5-Year Science Review (Presenter): Q3 ŌĆō How can GFDL research and modeling be further utilized to meet NOAA stakeholder needs and enhance research partnerships to ensure GFDLŌĆÖs success?, NOAA GFDL, NJ.
References...
Schreck III, C.M., D.R. Easterling, J.J. Barsugli, D.A. Coates, A. Hoell, N.C. Johnson, K.E. Kunkel, Z.M. Labe, J. Uehling, R.S. Vose, and X. Zhang (2024). A rapid response process for evaluating causes of extreme temperature events in the United States: the 2023 Texas/Louisiana heatwave as a prototype. Environmental Research: Climate, DOI:10.1088/2752-5295/ad8028
Zhang, Y., B.M. Ayyub, J.F. Fung, and Z.M. Labe (2024). Incorporating extreme event attribution into climate change adaptation for civil infrastructure: Methods, benefits, and research needs. Resilient Cities and Structures, DOI:10.1016/j.rcns.2024.03.002
Eischeid, J.K., M.P. Hoerling, X.-W. Quan, A. Kumar, J. Barsugli, Z.M. Labe, K.E. Kunkel, C.J. Schreck III, D.R. Easterling, T. Zhang, J. Uehling, and X. Zhang (2023). Why has the summertime central U.S. warming hole not disappeared? Journal of Climate, DOI:10.1175/JCLI-D-22-0716.1AUTOSOMES , ALLOSOMES AND SEX RATIO IN HUMAN POPULATION



AUTOSOMES , ALLOSOMES AND SEX RATIO IN HUMAN POPULATIONNistarini College, Purulia (W.B) India
╠²
This presentation offers a bird's eye view of autosomes and sex chromosomes. It also explores the different kinds of diseases of humans due to autosomal and sex-linked inherited traits. The sex determination of plants has been explained. The ratio of sex in the human population along with cause and consequences has been explained here.INHALANT_ANAESTHETICS_USED_IN_VETRINARY_PRACTICE.pptx



INHALANT_ANAESTHETICS_USED_IN_VETRINARY_PRACTICE.pptxrajvet4163
╠²
DESCRIBES INHALANT ANAESTHESIA USED FOR ANIMALS IN A NORMAL HOSPITAL SETUP2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...



2025-03-03-Data-related-Ethics Issues in Technologies for Professional Learni...Graz University of Technology & Know-Center
╠²
Unjustly Incriminating Bacteria: the Role of Bacteriophages in Bacterial Infe...



Unjustly Incriminating Bacteria: the Role of Bacteriophages in Bacterial Infe...christianagboeze2427
╠²
PROPERTIES-OF-MATTER chemistry lesson q1
- 1. Properties of Matter Use properties of matter to identify substances and to separate them, (STEM_GC11MP1a-b-5).
- 2. OBJECTIVES ŌĆó Discuss the physical and chemical properties of matter, such as density, boiling point, and solubility and relate how these properties can be used to identify substances. ŌĆó Identify common chemical apparatus and glassware. ŌĆó Demonstrate simple experiments to separate mixtures of substances using techniques such as filtration, distillation, and chromatography.
- 3. How can the following components of the following mixtures be separated? ŌĆóSalt from salt water ŌĆóSalt from a mixture of iron and salt
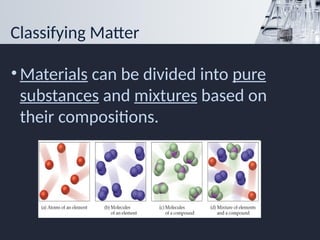
- 4. Classifying Matter ŌĆó Materials can be divided into pure substances and mixtures based on their compositions.
- 5. Pure Substances ŌĆó Matter that has exactly the same composition. ŌĆōhas the same properties because a substance has a uniform composition. ŌĆó Substances can be classified as elements or compounds.
- 6. Match the boxes at the left with the descriptions given below: 1.Element 2.Compound 3.Mixture of elements 4.Mixture of compounds 5.Mixture of compound and elements
- 7. Elements An element is a substance that cannot be broken down into simpler substances. An atom is the smallest particle of an element. An element has a fixed composition because it contains only one type of atom.
- 8. Elements ŌĆó Most elements are solids at room temperature. ŌĆó Some elements are gases at room temperature. Most of them are located on the upper right-hand side or the periodic table. ŌĆó Mercury and bromine are liquids at room temperature.
- 9. Symbols for Elements ŌĆó Each element symbol is either one or two letters. ŌĆó The first letter is always capitalized. If there is a second letter, it is lowercase. ŌĆó Some element symbols are based on the Latin names for elements. Ex: aurum = gold (Au) ferrum = iron (Fe)
- 10. Compounds ŌĆó A compound is a substance that is made of two or more simpler substances. ŌĆōproperties of compounds differ from those of the substances from which it is made ŌĆó A compound always contains two or more elements joined in a fixed proportion.
- 11. Mixtures ŌĆó The properties of a mixture can vary because the composition of a mixture is not fixed. ŌĆó Mixtures tend to retain some of the properties of their individual substances.
- 12. Heterogeneous Mixtures ŌĆó In a heterogeneous mixture, the parts of the mixture are noticeably different from one another.
- 13. Homogeneous Mixtures ŌĆó In a homogeneous mixture, the substances are so evenly distributed that it is difficult to distinguish one substance from another, so it appears to be uniform.
- 14. ŌĆó MATTER ŌĆó MIXTURE ŌĆó COMPOUND ŌĆó PURE SUBSTANCES ŌĆó HOMOGENOUS ŌĆó HETEREGOUS ŌĆó ELEMENT
- 17. Mixtures ŌĆó Based on the size of its largest particles, a mixture can be classified as a solution, a suspension, or a colloid.
- 20. Review _______________( L O N U T O S I ) When substances dissolve and form a homogeneous mixture, the mixture is called. _______________(O S N U E S I N S P) When a solution is a heterogeneous mixture that separates into layers over time.
- 21. ___________________( O D L L C O I) When a mixture contains particles that are intermediate in size, it does not settle, cannot be filtered, scatter light, and have medium-sized particles.
- 22. Solutions ŌĆó When substances dissolve and form a homogeneous mixture, the mixture that forms is called a solution. ŌĆó Properties of solutions: do not settle, cannot be filtered, allow light to pass through, and have small particles. ŌĆó Ex: windex, grape juice, and gasoline
- 23. Suspensions ŌĆó A suspension is a heterogeneous mixture that separates into layers over time. ŌĆó Properties of suspensions: settle over time, can be filtered, scatter light, and have large particles. ŌĆó Ex: Italian salad dressing, muddy water, and paint.
- 24. Colloids ŌĆó A colloid contains some particles that are intermediate in size. ŌĆó Properties of colloids: do not settle, cannot be filtered, scatter light, and have medium-sized particles. ŌĆó Ex: milk, ink, and Jell-O
- 25. ŌĆó Why does every sample of a given substance have the same properties? ŌĆó Explain why the composition of an element is fixed. ŌĆó Describe the composition of a compound. ŌĆó Why can the properties of a mixture vary? ŌĆó On what basis can mixtures be classified as solutions, suspensions, or colloids?
- 26. ŌĆó Explain why silicon dioxide cannot be the only compound in a sample of sand. ŌĆó Fresh milk is a suspension. After fresh milk is homogenized, it is a colloid. What happens to the size of the drops of fat in milk when it is homogenized?
- 27. 1.What are the 2 categories of matter? 2.What is an example of an element? 3.Is apple juice an example of a homogeneous or a heterogeneous mixture?
- 28. Physical Properties ŌĆó A physical property is any characteristic of a material that can be observed or measured without changing the composition of the substances in the material. ŌĆó Viscosity, conductivity, malleability, hardness, melting point, boiling point, and density
- 29. Viscosity ŌĆó The tendency of a liquid to keep from flowing ŌĆō its resistance to flowing ŌĆō is called its viscosity. ŌĆōThe greater the viscosity, the slower the liquid moves. ŌĆōThe viscosity of a liquid usually decreases when it is heated.
- 30. Conductivity ŌĆó A materialŌĆÖs ability to allow heat or energy to flow is called conductivity. ŌĆó Materials that have a high conductivity, such as metals, are called conductors.
- 31. Malleability ŌĆó Malleability is the ability of a solid to be hammered without shattering. ŌĆó Most metals are malleable. ŌĆó Solids that shatter when struck are brittle.
- 32. Hardness ŌĆóOne way to compare the hardness of two materials is to see which of the materials can scratch the other. ŌĆóDiamond is the hardest known material.
- 34. Melting and Boiling Points ŌĆó The temperature at which a substance changes from a solid to a liquid is its melting point. ŌĆó The temperature at which a substanceŌĆÖs internal pressure equals external pressure is its boiling point.
- 35. Density ŌĆóDensity is the ratio of the mass of a substance to its volume.
- 36. Physical Properties ŌĆó Physical properties are used to identify a material, to choose a material for a specific purpose, or to separate the substances in a mixture.
- 37. Physical Changes ŌĆó A physical change occurs when some of the properties of a material change, but the substances in the material remain the same. ŌĆó Physical changes can be reversible or irreversible. melting ice cutting paper
- 38. Chemical Properties ŌĆó A chemical property is any ability to produce a change in the composition of matter. ŌĆó Can be observed only when the substances in a sample of matter are changing into different substances. ŌĆó Flammability and reactivity are two examples of chemical properties.
- 39. Flammability ŌĆóFlammability is the ability to burn in the presence of oxygen.
- 40. Reactivity ŌĆóThe property that describes how readily a substance combines chemically with other substances is reactivity.
- 41. Chemical Changes ŌĆó A chemical change occurs when a substance reacts and forms one or more new substances. ŌĆó Three common types of evidence for a chemical change are a change in color, the production of a gas, and the formation of a precipitate.
- 42. Change in Color
- 43. Production of a Gas
- 44. Formation of a Precipitate ŌĆóAny solid that forms and separates from a liquid mixture is called a precipitate.







