Inflammatory Localized and Generalized Bone Loss in Recent-onset Rheumatoid Arthritis
- 1. el t uks h ig r-Y r y le p u o G C le k Me D r.
- 2. el t uks h ig r-Y r y le p u o G C le k Me D r.
- 3. el t uk s h ig r-Y Inflammatoir botverlies in vroege r y le reumatoïde artritis (RA) p u o G C le k MeInflammatoir botverlies in artrose r. (osteoarthritis=OA) D
- 4. Osteoporose en verlies aan botmineraaldichtheid Osteoporose el t uk s Verlies aan botmineraaldichtheid (BMD) h ig r-Y r 2 vormen van osteoporose / BMD verlies y le - gegeneraliseerd: door het hele skelet (heup, LWK) p u o G - gelokaliseerd: in directe nabijheid van gewrichten (hand) Cheup en onderrug (LWK) (DEXA) in de le k M e D r.
- 5. Osteoporose / BMD verlies in RA: wat was bekend? l 2x meer gegeneraliseerde osteoporose in pati├½nten met langbestaande RA, e resulterend in 2x vaker botbreuken. t uk s h ig r-Y 1. Overlappende risicofactoren: hogere leeftijd, (postmenopauzale) vrouwen, roken. r y le p u 2. Hoge ziekteactiviteit & gewrichtsschade o G Ōåō C immobilisatie / laag gewicht BMD verlies le k 3. Me Prednison gebruik ŌåÆ BMD verlies r. 4. Inflammatie ŌåÆ BMD verlies? D
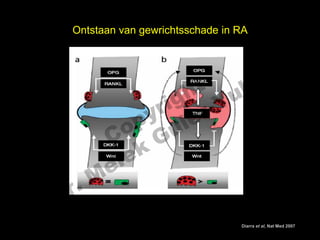
- 6. Ontstaan van gewrichtsschade in RA el t uk s h ig r-Y r y le p u o G C le k Me D r. Leidt zelfde mechanisme tot BMD verlies en osteoporose? Diarra et al, Nat Med 2007
- 7. Osteoporose / BMD verlies in RA: wat was onbekend? 1. el Wat is de mate van gelokaliseerd en gegeneraliseerd osteoporose / BMD verlies s in pas gediagnosticeerde RA? h t uk ig r-Y 2. Wordt BMD verlies direct veroorzaakt door inflammatoir activiteit in RA? r y le p u 3. Wat is het effect van hedendaagse, moderne behandelstrategieën op BMD verlies o G in RA? C le k Me D r.
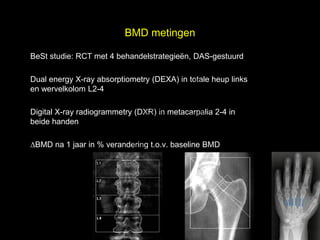
- 8. BMD metingen l BeSt studie: RCT met 4 behandelstrategie├½n, DAS-gestuurd t uk Dual energy X-ray absorptiometry (DEXA) in totale heup links s e en wervelkolom L2-4 h ig r-Y r y le p u Digital X-ray radiogrammetry (DXR) in metacarpalia 2-4 in o G beide handen C k ŌłåBMD na 1 jaar in % verandering t.o.v. baseline BMD le Me D r.
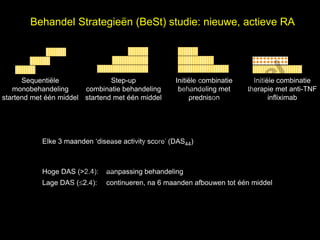
- 9. Behandel Strategie├½n (BeSt) studie: nieuwe, actieve RA el t uk s h Sequenti├½le Step-up Initi├½le combinatie Initi├½le combinatie ig r-Y monobehandeling combinatie behandeling behandeling met therapie met anti-TNF r startend met ├®├®n middel startend met ├®├®n middel prednison infliximab y le p u C o G k Elke 3 maanden ŌĆśdisease activity scoreŌĆÖ (DAS44) e le M Hoge DAS (>2.4): aanpassing behandeling r. Lage DAS (’éŻ2.4): continueren, na 6 maanden afbouwen tot ├®├®n middel D
- 10. Baseline karakteristieken (n=218) Vrouw, % 71 Postmenopausaal, % 65 el Leeftijd, jaren t uk 54 s Symptoomduur, weken h ig r-Y 23 r DAS44 4.4 RF +, % y le p u 64 C o G Erosieve ziekte, % 69 le k Me D r.
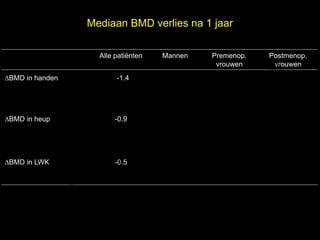
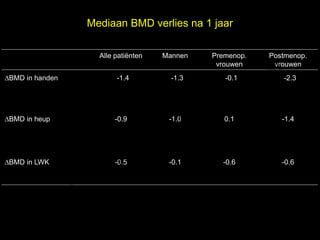
- 11. Mediaan BMD verlies na 1 jaar l Alle pati├½nten Mannen Premenop. Postmenop. e vrouwen vrouwen ŌłåBMD in handen -1.4 t uk -1.3 -0.1 s -2.3 h ig r-Y r y le ŌłåBMD in heup p u o G -0.9 -1.0 0.1 -1.4 C le k ŌłåBMD in LWK Me -0.5 -0.1 -0.6 -0.6 D r.
- 12. Mediaan BMD verlies na 1 jaar l Alle pati├½nten Mannen Premenop. Postmenop. e vrouwen vrouwen ŌłåBMD in handen -1.4 t uk -1.3 -0.1 s -2.3 h ig r-Y r y le ŌłåBMD in heup p u o G -0.9 -1.0 0.1 -1.4 C le k ŌłåBMD in LWK Me -0.5 -0.1 -0.6 -0.6 D r.
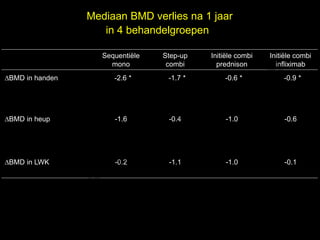
- 13. Mediaan BMD verlies na 1 jaar in 4 behandelgroepen l Sequenti├½le Step-up Initi├½le combi Initi├½le combi e mono combi prednison infliximab ŌłåBMD in handen -2.6 * t uk -1.7 * -0.6 * s -0.9 * h ig r-Y r y le ŌłåBMD in heup p u o G -1.6 -0.4 -1.0 -0.6 C le k ŌłåBMD in LWK Me -0.2 -1.1 -1.0 -0.1 D r.
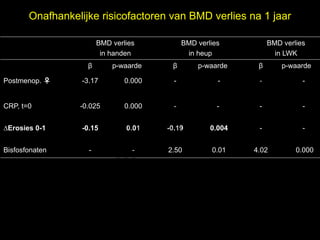
- 14. Onafhankelijke risicofactoren van BMD verlies na 1 jaar BMD verlies BMD verlies BMD verlies in handen in heup in LWK ╬▓ p-waarde ╬▓ p-waarde ╬▓ el p-waarde t uk s h Postmenop. ŌÖĆ -3.17 0.000 - - - - r ig r-Y y le CRP, t=0 -0.025 0.000 - - - - p u o G C ŌłåErosies 0-1 -0.15 0.01 -0.19 0.004 - - Bisfosfonaten - le k - 2.50 0.01 4.02 0.000 Me D r.
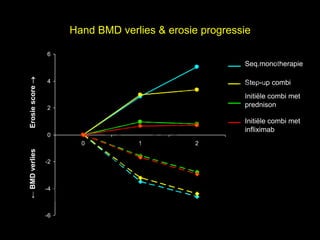
- 15. Hand BMD verlies & erosie progressie 6 el Seq.monotherapie t uk s Erosie score ’é« h 4 Step-up combi r ig r-Y Initi├½le combi met y le 2 prednison p u o G Initi├½le combi met C infliximab 0 k 0 1 2 le ŌåÉ BMD verlies e -2 M -4 D r. -6
- 16. Osteoporose / BMD verlies in RA: wat is er nu bekend? 1. el In pas gediagnosticeerde RA treedt meer lokaal BMD verlies in handen op dan s gegeneraliseerd BMD verlies in heup en LWK. h t uk ig r-Y 2. Progressieve erosieve gewrichtsschade is geassocieerd met meer lokaal en r gegeneraliseerd botverlies ’āĀ gedeelde mechanismes tussen deze processen. y le p u o G 3. Er is minder BMD verlies in handen in de initi├½le combinatie behandelgroepen C door effectievere suppressie van de inflammatie in de eerste 6 maanden. le k Me D r.
- 17. el t uk s h ig r-Y Is lokaal BMD verlies een voorspeller voor destructie in r y le pas gediagnosticeerde RA? p u o G C le k Me D r.
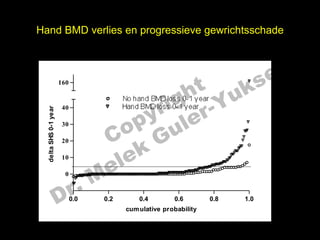
- 18. Hand BMD verlies en progressieve gewrichtsschade el t uk s h 160 r ig r-Y No hand BMD loss 0-1 year y le Hand BMD loss 0-1 year 40 delta SHS 0-1 year p u o G C 30 k 20 10 e le M ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ r. 0 D 0.0 0.2 0.4 0.6 cum ulative probability 0.8 1.0
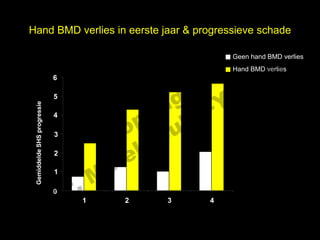
- 19. Hand BMD verlies in eerste jaar & progressieve schade l Geen hand BMD verlies s e Hand BMD verlies t uk 6 h ig r-Y r 5 y le Gemiddelde SHS progressie p u 4 3 C o G 2 le k Me r. 1 D 0 1 2 3 4 follow-up, jaren
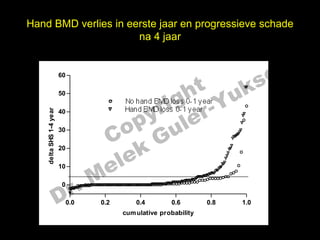
- 20. Hand BMD verlies in eerste jaar en progressieve schade na 4 jaar el t uk s 60 h 160 ig r-Y 50 r No hand BMD loss 0-1 year y le Hand BMD loss 0-1 year p u 1-4 year 40 delta SHS 0-1 year 40 30 30 C o G k 20 le 20 delta e 10 M 10 ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ r. 0 ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ’ĆŁ ’ĆŁ’ĆŁ’ĆŁ’ĆŁ D 0 0.0 0.0 0.2 0.2 0.4 0.4 0.6 0.6 0.8 0.8 1.0 1.0 cum ulative probability cum ulative probability
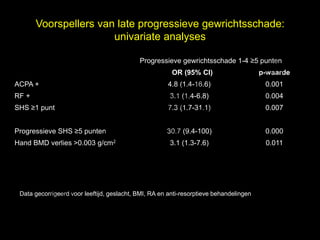
- 21. Voorspellers van late progressieve gewrichtsschade: univariate analyses l Progressieve gewrichtsschade 1-4 Ōēź5 punten t uk OR (95% CI) s e p-waarde h ACPA + 4.8 (1.4-16.6) 0.001 r ig r-Y RF + 3.1 (1.4-6.8) 0.004 y le SHS Ōēź1 punt 7.3 (1.7-31.1) 0.007 p u o G C Progressieve SHS Ōēź5 punten 30.7 (9.4-100) 0.000 k Hand BMD verlies >0.003 g/cm2 3.1 (1.3-7.6) 0.011 e le M D r. Data gecorrigeerd voor leeftijd, geslacht, BMI, RA en anti-resorptieve behandelingen
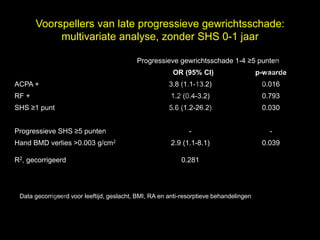
- 22. Voorspellers van late progressieve gewrichtsschade: multivariate analyse, zonder SHS 0-1 jaar l Progressieve gewrichtsschade 1-4 Ōēź5 punten t uk OR (95% CI) s e p-waarde h ACPA + 3.8 (1.1-13.2) 0.016 r ig r-Y RF + 1.2 (0.4-3.2) 0.793 y le SHS Ōēź1 punt 5.6 (1.2-26.2) 0.030 p u o G C Progressieve SHS Ōēź5 punten - - k Hand BMD verlies >0.003 g/cm2 2.9 (1.1-8.1) 0.039 R2, gecorrigeerd e le 0.281 M D r. Data gecorrigeerd voor leeftijd, geslacht, BMI, RA en anti-resorptieve behandelingen
- 23. Voorspellers van late progressieve gewrichtsschade: multivariate analyse, met SHS 0-1 jaar l Progressieve gewrichtsschade 1-4 Ōēź5 punten t uk OR (95% CI) s e p-waarde h ACPA + 3.5 (1.7-7.5) 0.013 r ig r-Y RF + 1.7 (0.5-5.9) 0.386 y le SHS Ōēź1 punt 2.2 (0.4-12.0) 0.363 p u o G C Progressieve SHS Ōēź5 punten 29.2 (11.8-72.4) 0.000 k Hand BMD verlies >0.003 g/cm2 1.4 (0.4-4.4) 0.603 R2, gecorrigeerd e le 0.514 M D r. Data gecorrigeerd voor leeftijd, geslacht, BMI, RA and anti-resorptieve behandelingen
- 24. BMD verlies voorspelt gewrichtsschade in RA l Hand BMD verlies in eerste jaar is geassocieerd met late progressieve gewrichtsschade, echter niet onafhankelijk van progressieve gewrichtsschade in eerste jaar. t uk s e h ig r-Y r Hand BMD verlies na 1 jaar is als voorspeller klinisch niet relevant, data over hand BMD y le verlies na 3 a 4 maanden zijn onderweg. p u o G C le k Me D r.
- 25. el t uks BMD verliesgh ri r-Y in OA p uy le C o G le k Me D r.
- 26. Pathogenese OA el Multifactorieel: s Degeneratieve factoren Biomechanische factoren h t uk ig r-Y Metabole factoren Hormonale factoren r y le p u Genetische factoren o G Inflammatoire factoren? C le k Me D r.
- 27. Inflammatie in OA el Meer evidence dat lokale en low-grade systemische inflammatie rol speelt in s ontwikkeling en progressie OA h t uk - Verhoogde levels pro-inflammatoire cytokines in gewrichtsvocht ig r-Y - Verhoogde levels (high sensitive) CRP r - (High resolution) MRI: tekenen van inflammatie (subchondrale botoedeem, y le p u erosies in interphalangeale gewrichten) C o G le k Me D r.
- 28. GARP (Genetics, arthrosis, progression) studie l 181 pati├½nten met OA t uk Radiologische schade 0-2 jaar in IPJs en CMC1 s e h - Baseline: Kellgren-Lawrence score (0-4 punten per gewricht): KL Ōēź2 punten in Ōēź2 ig r-Y gewrichten = hand OA r - Progressie: OARSI atlas: Ōēź1 punt (=SDC) bij pati├½nten met hand OA op baseline y le p u o G Hand DXR-BMD verlies 0-2 jaar C le k Vraag: Is progressieve hand OA geassocieerd met meer hand BMD verlies? Me D r.
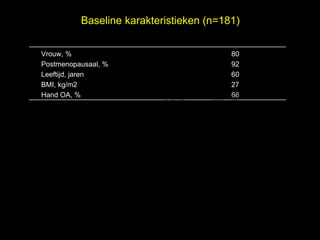
- 29. Baseline karakteristieken (n=181) Vrouw, % 80 Postmenopausaal, % 92 el t uk s Leeftijd, jaren 60 h BMI, kg/m2 27 ig r-Y Hand OA, % 68 r y le p u o G C le k Me D r.
- 30. BMD verlies in OA el Progressieve hand Non-progressieve hand Geen hand OA s OA OA h Groep 1 n=39 t uk Groep 2 n=84 Groep 3 n=58 Mediaan (IQR) ΔBMD r ig r-Y y le -2.2% -1.4% -1.4% p u 0-2 jaar (-4.1 tot -1.4) (-2.9 tot -0.6) (-3.1 tot -0.4) C o G P-waarde, overall: 0.032 le k Groep 1 vs 2: 0.045 Me r. Groep 2 vs 3: 0.804 D
- 31. BMD verlies in OA RR (95% CI) el t uk s Geen hand OA Progressieve hand OA BMD verlies 0-2 jaar 1 h ig r-Y 2.1 (1.1-4.3) r y le p u o G C le k Me Multivariate analyses gecorrigeerd voor leeftijd, geslacht, postmenopausale status, BMI, r. familiair effect, roken, gebruik anti-osteoporose med en BMD scores op baseline D
- 32. BMD verlies in OA l Lokaal BMD verlies in handen is geassocieerd met (progressieve) hand OA na 2 e t uk s jaar. h ig r-Y r Pathogenese van OA is multifactorieel, mogelijk speelt (lokaal) inflammatoir activiteit ook een rol. y le p u C o G le k Me D r.
- 33. el t uks IWO, dank voorgh prijs ri r-Y HvP p uy le C o G le k M e D r.