Lecture 03_Metal structure and Crystallization.pptx
- 1. Engineering Materials ME 1113 Lecture - 03 Metal structure and Crystallization Prepared by Md. Helal Hossain
- 2. Crystal Geometry To study the crystal geometry, we have to have knowledge about following topics. ŌĆó Crystal. ŌĆó Lattice. ŌĆó Motif. ŌĆó Unit cell. ŌĆó 7 crystal Systems. ŌĆó 14 Bravais lattice. ŌĆó Miller Indices. ME 1113 MD. HELAL HOSSAIN ENGINEERING MATERIALS
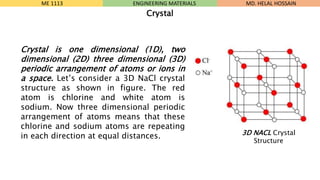
- 3. Crystal Crystal is one dimensional (1D), two dimensional (2D) three dimensional (3D) periodic arrangement of atoms or ions in a space. LetŌĆÖs consider a 3D NaCl crystal structure as shown in figure. The red atom is chlorine and white atom is sodium. Now three dimensional periodic arrangement of atoms means that these chlorine and sodium atoms are repeating in each direction at equal distances. ME 1113 ENGINEERING MATERIALS 3D NACL Crystal Structure MD. HELAL HOSSAIN
- 4. Lattice Lattice is one dimensional (1D), two dimensional (2D) three dimensional (3D) periodic arrangement of points in a space. The difference between crystal and lattice is whether the points are being considered or atoms are being considered otherwise both are one and three dimensional periodic arrangement. ME 1113 ENGINEERING MATERIALS Infinite lattice MD. HELAL HOSSAIN
- 5. Crystal and Lattice Difference: Relation between crystal and lattice: The relationship between them is expressed by following equation. Crystal = Lattice + Motif or Basis So the linking bridge between crystal and lattice is a motif. ME 1113 ENGINEERING MATERIALS MD. HELAL HOSSAIN
- 6. Lattice Translation: Lattice translation is defined as any vector from one lattice point to another lattice point. In the figure, blue color arrow indicates the lattice translation but red color arrow does not represent lattice translation since there is one lattice point in-between first and last lattice point. ME 1113 ENGINEERING MATERIALS Lattice MD. HELAL HOSSAIN
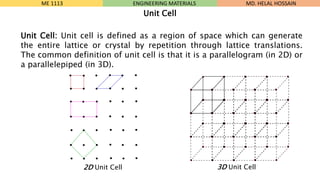
- 7. Unit Cell: Unit cell is defined as a region of space which can generate the entire lattice or crystal by repetition through lattice translations. The common definition of unit cell is that it is a parallelogram (in 2D) or a parallelepiped (in 3D). ME 1113 ENGINEERING MATERIALS Unit Cell 2D Unit Cell 3D Unit Cell MD. HELAL HOSSAIN
- 8. Based on the unit cell geometry, there are seven basic crystal system: ME 1113 ENGINEERING MATERIALS 7 Crystal System Tetragonal Orthorhombic Monoclinic Triclinic Trigonal Hexagonal Cubic MD. HELAL HOSSAIN
- 9. ME 1113 ENGINEERING MATERIALS 14 Bravais Lattice Systems Bravais Lattice refers to the 14 different 3- dimensional configurations into which atoms can be arranged in crystals. They are: Cubic System: In Bravais lattices with cubic systems, the following relationships can be observed. a = b = c Øøé = Ø×½ = ØØ▓ = 90┬░ The 3 possible types of cubic cells. Examples: Polonium has a simple cubic structure, iron has a body-centered cubic structure, and copper has a face-centered cubic structure. MD. HELAL HOSSAIN
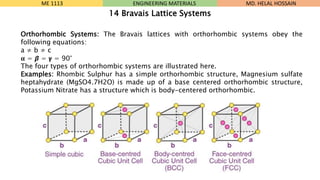
- 10. Orthorhombic Systems: The Bravais lattices with orthorhombic systems obey the following equations: a ŌēĀ b ŌēĀ c Øøé = Ø×½ = ØØ▓ = 90┬░ The four types of orthorhombic systems are illustrated here. Examples: Rhombic Sulphur has a simple orthorhombic structure, Magnesium sulfate heptahydrate (MgSO4.7H2O) is made up of a base centered orthorhombic structure, Potassium Nitrate has a structure which is body-centered orthorhombic. ME 1113 ENGINEERING MATERIALS 14 Bravais Lattice Systems MD. HELAL HOSSAIN
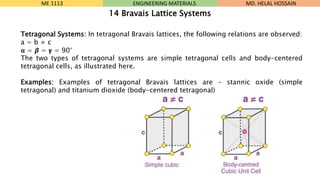
- 11. Tetragonal Systems: In tetragonal Bravais lattices, the following relations are observed: a = b ŌēĀ c Øøé = Ø×½ = ØØ▓ = 90┬░ The two types of tetragonal systems are simple tetragonal cells and body-centered tetragonal cells, as illustrated here. Examples: Examples of tetragonal Bravais lattices are ŌĆō stannic oxide (simple tetragonal) and titanium dioxide (body-centered tetragonal) ME 1113 ENGINEERING MATERIALS 14 Bravais Lattice Systems MD. HELAL HOSSAIN
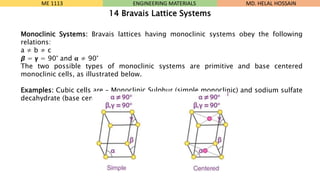
- 12. Monoclinic Systems: Bravais lattices having monoclinic systems obey the following relations: a ŌēĀ b ŌēĀ c Ø×½ = ØØ▓ = 90┬░ and Øøé ŌēĀ 90┬░ The two possible types of monoclinic systems are primitive and base centered monoclinic cells, as illustrated below. Examples: Cubic cells are ŌĆō Monoclinic Sulphur (simple monoclinic) and sodium sulfate decahydrate (base centered monoclinic) ME 1113 ENGINEERING MATERIALS 14 Bravais Lattice Systems MD. HELAL HOSSAIN
- 13. Triclinic System: There exists only one type of triclinic Bravais lattice, which is a primitive cell. It obeys the following relationship. a ŌēĀ b ŌēĀ c Øøé ŌēĀ Ø×½ ŌēĀ ØØ▓ ŌēĀ 90┬░ Examples: potassium dichromate (Chemical formula K2Cr2O7). ME 1113 ENGINEERING MATERIALS 14 Bravais Lattice Systems Rhombohedral System: Only the primitive unit cell for a rhombohedral system exists. Its cell relation is given by: a = b = c Øøé = Ø×½ = ØØ▓ ŌēĀ 90┬░ Examples: Calcite and sodium nitrate are made up of simple rhombohedral unit cells. MD. HELAL HOSSAIN
- 14. Hexagonal System: The only type of hexagonal Bravais lattice is the simple hexagonal cell. It has the following relations between cell sides and angles. a = b ŌēĀ c Øøé = Ø×½ = 90o and ØØ▓ = 120┬░ An illustration of a simple hexagonal cell is provided below. Examples: Zinc oxide and beryllium oxide are made up of simple hexagonal unit cells. Thus, it can be noted that all 14 possible Bravais lattices differ in their cell length and angle relationships. It is important to keep in mind that the Bravais lattice is not always the same as the crystal lattice. ME 1113 ENGINEERING MATERIALS 14 Bravais Lattice Systems MD. HELAL HOSSAIN
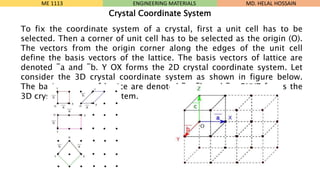
- 15. To fix the coordinate system of a crystal, first a unit cell has to be selected. Then a corner of unit cell has to be selected as the origin (O). The vectors from the origin corner along the edges of the unit cell define the basis vectors of the lattice. The basis vectors of lattice are denoted ¯a and ¯b. Y OX forms the 2D crystal coordinate system. Let consider the 3D crystal coordinate system as shown in figure below. The basis vectors of lattice are denoted ¯a, ¯b and ¯c. OXYZ forms the 3D crystal coordinate. system. ME 1113 ENGINEERING MATERIALS Crystal Coordinate System MD. HELAL HOSSAIN
- 16. Lattice Parameters: The lengths of two edges of unit cell or basis vectors (in 2D unit cell) and the lengths of three edges of unit cell or basis vectors (in 3D unit cell); and the inter-axial angles between basis vectors are defined as the lattice parameters. ŌĆó Lattice Parameters of 2D Unit Cells: In case of 2D unit cells, the number of lattice parameters are three: two length of edges and one angle between them. They are denoted as a = length of edge b = length of edge ╬│ = angle between two a and b These parameters in different 2D unit cells are shown in figure ME 1113 ENGINEERING MATERIALS Lattice Parameters MD. HELAL HOSSAIN
- 17. ŌĆó Lattice Parameters of 3D Unit Cells: In case of 3D unit cells, the number of lattice parameters are six: three length of edges and three angles between them. They are denoted as a┬» = length of edge ┬»b = length of edge c┬» = length of edge ╬│ = angle between two ┬» a and ┬»b ╬▓ = angle between two ┬» a and ┬» c ╬▒ = angle between two ┬»b and ┬» c These parameters in different 3D unit cells are shown in figure. ME 1113 ENGINEERING MATERIALS Lattice Parameters MD. HELAL HOSSAIN
- 18. In a crystal lattice, planes are defined by the arrangement of atoms, and directions are defined by the path between atoms. Miller indices provide a concise and systematic way to describe these planes and directions. To determine the Miller indices of a plane, you take the reciprocals of the intercepts made by the plane on the crystallographic axes and multiply them by a common denominator to get integer values. The resulting integers are then enclosed in parentheses, written as (hkl) and for the direction it is written as [hkl] ME 1113 ENGINEERING MATERIALS Miller Indices MD. HELAL HOSSAIN
- 19. Why is Miller indices important: ŌĆó Miller indices play a crucial role in crystallography as they help in identifying and characterizing different crystallographic planes and directions. ŌĆó They provide a universal language for scientists to communicate and understand the structural properties of crystals, enabling the study of various physical and chemical phenomena in materials science, solid- state physics, and mineralogy. ME 1113 ENGINEERING MATERIALS Miller Indices MD. HELAL HOSSAIN
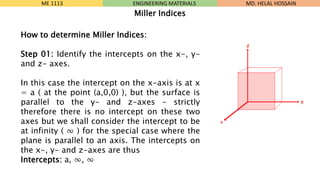
- 20. How to determine Miller Indices: Step 01: Identify the intercepts on the x-, y- and z- axes. In this case the intercept on the x-axis is at x = a ( at the point (a,0,0) ), but the surface is parallel to the y- and z-axes - strictly therefore there is no intercept on these two axes but we shall consider the intercept to be at infinity ( Ōł× ) for the special case where the plane is parallel to an axis. The intercepts on the x-, y- and z-axes are thus Intercepts: a, Ōł×, Ōł× ME 1113 ENGINEERING MATERIALS Miller Indices MD. HELAL HOSSAIN
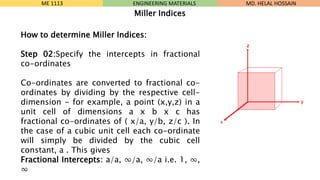
- 21. How to determine Miller Indices: Step 02:Specify the intercepts in fractional co-ordinates Co-ordinates are converted to fractional co- ordinates by dividing by the respective cell- dimension - for example, a point (x,y,z) in a unit cell of dimensions a x b x c has fractional co-ordinates of ( x/a, y/b, z/c ). In the case of a cubic unit cell each co-ordinate will simply be divided by the cubic cell constant, a . This gives Fractional Intercepts: a/a, Ōł×/a, Ōł×/a i.e. 1, Ōł×, Ōł× ME 1113 ENGINEERING MATERIALS Miller Indices MD. HELAL HOSSAIN
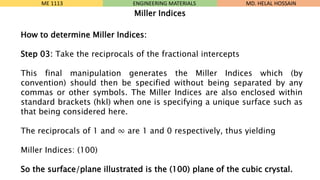
- 22. How to determine Miller Indices: Step 03: Take the reciprocals of the fractional intercepts This final manipulation generates the Miller Indices which (by convention) should then be specified without being separated by any commas or other symbols. The Miller Indices are also enclosed within standard brackets (hkl) when one is specifying a unique surface such as that being considered here. The reciprocals of 1 and Ōł× are 1 and 0 respectively, thus yielding Miller Indices: (100) So the surface/plane illustrated is the (100) plane of the cubic crystal. ME 1113 ENGINEERING MATERIALS Miller Indices MD. HELAL HOSSAIN
- 23. ME 1113 ENGINEERING MATERIALS Miller Indices - Examples MD. HELAL HOSSAIN
- 24. ME 1113 ENGINEERING MATERIALS Miller Indices - Examples MD. HELAL HOSSAIN
- 25. ME 1113 ENGINEERING MATERIALS Miller Indices - Examples MD. HELAL HOSSAIN
- 26. ME 1113 ENGINEERING MATERIALS Miller Indices - Examples DO IT YOURSEL F MD. HELAL HOSSAIN
- 27. ME 1113 ENGINEERING MATERIALS Miller Indices - Examples DO IT YOURSEL F MD. HELAL HOSSAIN
- 28. ME 1113 ENGINEERING MATERIALS Polymorphism and Allotropy Polymorphism: Polymorphism in general means having many forms. The term is used in chemistry to describe the ability of a chemical compound to crystallize in multiple unit cell configurations. Types of polymorphism: 1. Monotropic System: In a monotropic system, only one polymorph is stable at all temperature ranges. A very good example of a monotropic system is metolazone. 2. Enantiotropic System: In an enantiotropic system, different polymorphs are stable across different temperature ranges. Examples of an enantiotropic system include carbamazepine and acetazolamide. MD. HELAL HOSSAIN
- 29. ME 1113 ENGINEERING MATERIALS Polymorphism and Allotropy Allotropy: While polymorphism is the ability of a solid material to exist in more than one crystal structure, allotropy is the ability of chemical elements to exist in two or more different forms in the same physical state. Difference between polymorphism and allotropy: Polymorphism is the ability of a solid material to exist in more than one crystal structure. Allotropy is the ability of chemical elements to exist in two or more different forms in the same physical state. In the case of crystal solids, allotropy is a particular case of polymorphism. MD. HELAL HOSSAIN
- 30. End of Lecture ŌĆō 03 Thank you ME 1113 ENGINEERING MATERIALS MD. HELAL HOSSAIN










![In a crystal lattice, planes are defined by
the arrangement of atoms, and
directions are defined by the path
between atoms. Miller indices provide a
concise and systematic way to describe
these planes and directions.
To determine the Miller indices of a
plane, you take the reciprocals of the
intercepts made by the plane on the
crystallographic axes and multiply them
by a common denominator to get integer
values. The resulting integers are then
enclosed in parentheses, written as (hkl)
and for the direction it is written as [hkl]
ME 1113 ENGINEERING MATERIALS
Miller Indices
MD. HELAL HOSSAIN](https://image.slidesharecdn.com/lecture03metalstructureandcrystallization-240713141519-3b3b8692/85/Lecture-03_Metal-structure-and-Crystallization-pptx-18-320.jpg)