1 of 11
Downloaded 10 times










Recommended
Solubility



SolubilityNinth Grade
?
Solubility is defined as the maximum amount of solute that can dissolve in a specific amount of solvent, usually expressed as grams of solute per 100 grams of solvent. Unsaturated solutions contain less than the maximum amount of solute and can dissolve more, while saturated solutions contain the maximum amount of solute with some remaining undissolved at the bottom of the container. The solubility of most solids increases with increasing temperature, while the solubility of gases decreases with increasing temperature.The Preparation Of Potash Alum



The Preparation Of Potash AlumCool Guy
?
Preparation of potash alum from scrap aluminium.
Very good topic for class 12th chemistry project work.
Acids, bases + neutralization



Acids, bases + neutralizationMahesh Rathva
?
neutralization (or neutralisation, see spelling differences) is a chemical reaction in which an acid and a base react to form a salt. Water is frequently, but not necessarily, produced as well. Neutralizations with Arrhenius acids and bases always produce water where acid–alkali reactions produce water and a metal salt.IB Chemistry on Gibbs Free Energy and Equilibrium constant, Kc



IB Chemistry on Gibbs Free Energy and Equilibrium constant, KcLawrence kok
?
This document discusses chemical equilibrium, including the equilibrium constant Kc, factors that affect equilibrium, and the relationship between equilibrium and thermodynamics. At equilibrium, the forward and reverse reaction rates are equal, and the concentrations of reactants and products remain constant. The equilibrium constant Kc is defined as the ratio of product concentrations over reactant concentrations raised to their stoichiometric coefficients. An increase in temperature can shift the position of equilibrium either to the left or right depending on whether the reaction is exothermic or endothermic. The Gibbs free energy change ΔG is related to Kc and can be used to predict spontaneity. A more negative ΔG corresponds to a higher Kc and a greater extent of reaction towards productsChem 2 - Chemical Equilibrium III: The Equilibrium Constant Expression and th...



Chem 2 - Chemical Equilibrium III: The Equilibrium Constant Expression and th...Lumen Learning
?
Chem 2 - Chemical Equilibrium III: The Equilibrium Constant Expression and the Law of Mass Action (LOMA)#13 Key



#13 KeyLamar1411_SI
?
This document provides sample problems and questions for a general chemistry exam. It includes sample balanced equations for neutralization reactions and definitions of key terms like oxidation, reduction, concentration, and indicators. It also asks students to determine oxidation states, identify redox reactions, calculate molarity and amounts of substances in solutions, and determine concentrations of ions after mixing solutions.Chemisry project



Chemisry projectMIAMS, MANIPAL
?
Rajkira conducted a chemistry project to determine the contents of various cold drinks. Through a series of qualitative tests, Rajkira found that all the drinks contained glucose, alcohol, sucrose, phosphate, and carbon dioxide. The drinks varied in their acidity levels and amount of dissolved carbon dioxide. While cold drinks provide refreshment, they can also be harmful due to their sugar content and ability to damage teeth and bones over long-term consumption.Chapter 15 Lecture- Chemical Equilibrium



Chapter 15 Lecture- Chemical EquilibriumMary Beth Smith
?
1. The document discusses chemical equilibrium, including the concepts of equilibrium, depicting equilibrium reactions with equations, the equilibrium constant K, and how the value of K relates to whether a reaction favors reactants or products.
2. It also covers heterogeneous equilibria involving solids or liquids, how the concentrations of solids and liquids do not appear in equilibrium expressions, and examples of heterogeneous equilibrium reactions like the decomposition of calcium carbonate.
3. The key aspects covered are the definition of chemical equilibrium as when forward and reverse reactions proceed at the same rate, the use of concentration ratios and partial pressures to define equilibrium constants Kc and Kp, and how heterogeneous reactions involve gases in equilibrium with solids or liquids.AQA Chemistry C4 Answers Key



AQA Chemistry C4 Answers KeyPrawee Kaoklong
?
This document provides answers to chemical calculation problems from a student workbook. It includes:
1) Answers to questions about relative atomic masses, chemical equations, mass to mole conversions, reaction yields, atom economy, concentration expressions, titration procedures and calculations, gas volumes, and molar volume.
2) The answers are accompanied by guidance on the number of marks awarded for each part of the answers.
3) The resource may have been modified from the original but provides a comprehensive summary of answers to practice problems covering key topics in chemical calculations.8.1 the characteristic properties of acids and bases



8.1 the characteristic properties of acids and basesMuhammad Abdul Mageid
?
This document discusses acids, bases and salts. It defines acids as substances that produce hydrogen ions (H+) in water, giving them a pH below 7. Bases are defined as substances that produce hydroxide ions (OH-) in water, giving them a pH above 7. The document explains the characteristic properties of acids and bases, including their reactions with metals, metal oxides, carbonates and each other. It also discusses the pH scale, strong vs. weak acids and bases, and how concentration affects acid and base strength.More Related Content
What's hot (20)
Solubility



SolubilityNinth Grade
?
Solubility is defined as the maximum amount of solute that can dissolve in a specific amount of solvent, usually expressed as grams of solute per 100 grams of solvent. Unsaturated solutions contain less than the maximum amount of solute and can dissolve more, while saturated solutions contain the maximum amount of solute with some remaining undissolved at the bottom of the container. The solubility of most solids increases with increasing temperature, while the solubility of gases decreases with increasing temperature.The Preparation Of Potash Alum



The Preparation Of Potash AlumCool Guy
?
Preparation of potash alum from scrap aluminium.
Very good topic for class 12th chemistry project work.
Acids, bases + neutralization



Acids, bases + neutralizationMahesh Rathva
?
neutralization (or neutralisation, see spelling differences) is a chemical reaction in which an acid and a base react to form a salt. Water is frequently, but not necessarily, produced as well. Neutralizations with Arrhenius acids and bases always produce water where acid–alkali reactions produce water and a metal salt.IB Chemistry on Gibbs Free Energy and Equilibrium constant, Kc



IB Chemistry on Gibbs Free Energy and Equilibrium constant, KcLawrence kok
?
This document discusses chemical equilibrium, including the equilibrium constant Kc, factors that affect equilibrium, and the relationship between equilibrium and thermodynamics. At equilibrium, the forward and reverse reaction rates are equal, and the concentrations of reactants and products remain constant. The equilibrium constant Kc is defined as the ratio of product concentrations over reactant concentrations raised to their stoichiometric coefficients. An increase in temperature can shift the position of equilibrium either to the left or right depending on whether the reaction is exothermic or endothermic. The Gibbs free energy change ΔG is related to Kc and can be used to predict spontaneity. A more negative ΔG corresponds to a higher Kc and a greater extent of reaction towards productsChem 2 - Chemical Equilibrium III: The Equilibrium Constant Expression and th...



Chem 2 - Chemical Equilibrium III: The Equilibrium Constant Expression and th...Lumen Learning
?
Chem 2 - Chemical Equilibrium III: The Equilibrium Constant Expression and the Law of Mass Action (LOMA)#13 Key



#13 KeyLamar1411_SI
?
This document provides sample problems and questions for a general chemistry exam. It includes sample balanced equations for neutralization reactions and definitions of key terms like oxidation, reduction, concentration, and indicators. It also asks students to determine oxidation states, identify redox reactions, calculate molarity and amounts of substances in solutions, and determine concentrations of ions after mixing solutions.Chemisry project



Chemisry projectMIAMS, MANIPAL
?
Rajkira conducted a chemistry project to determine the contents of various cold drinks. Through a series of qualitative tests, Rajkira found that all the drinks contained glucose, alcohol, sucrose, phosphate, and carbon dioxide. The drinks varied in their acidity levels and amount of dissolved carbon dioxide. While cold drinks provide refreshment, they can also be harmful due to their sugar content and ability to damage teeth and bones over long-term consumption.Chapter 15 Lecture- Chemical Equilibrium



Chapter 15 Lecture- Chemical EquilibriumMary Beth Smith
?
1. The document discusses chemical equilibrium, including the concepts of equilibrium, depicting equilibrium reactions with equations, the equilibrium constant K, and how the value of K relates to whether a reaction favors reactants or products.
2. It also covers heterogeneous equilibria involving solids or liquids, how the concentrations of solids and liquids do not appear in equilibrium expressions, and examples of heterogeneous equilibrium reactions like the decomposition of calcium carbonate.
3. The key aspects covered are the definition of chemical equilibrium as when forward and reverse reactions proceed at the same rate, the use of concentration ratios and partial pressures to define equilibrium constants Kc and Kp, and how heterogeneous reactions involve gases in equilibrium with solids or liquids.AQA Chemistry C4 Answers Key



AQA Chemistry C4 Answers KeyPrawee Kaoklong
?
This document provides answers to chemical calculation problems from a student workbook. It includes:
1) Answers to questions about relative atomic masses, chemical equations, mass to mole conversions, reaction yields, atom economy, concentration expressions, titration procedures and calculations, gas volumes, and molar volume.
2) The answers are accompanied by guidance on the number of marks awarded for each part of the answers.
3) The resource may have been modified from the original but provides a comprehensive summary of answers to practice problems covering key topics in chemical calculations.8.1 the characteristic properties of acids and bases



8.1 the characteristic properties of acids and basesMuhammad Abdul Mageid
?
This document discusses acids, bases and salts. It defines acids as substances that produce hydrogen ions (H+) in water, giving them a pH below 7. Bases are defined as substances that produce hydroxide ions (OH-) in water, giving them a pH above 7. The document explains the characteristic properties of acids and bases, including their reactions with metals, metal oxides, carbonates and each other. It also discusses the pH scale, strong vs. weak acids and bases, and how concentration affects acid and base strength.Viewers also liked (11)
More from fbw41598 (20)
康轩中自二下笔辫迟经典款肠丑1重点整理
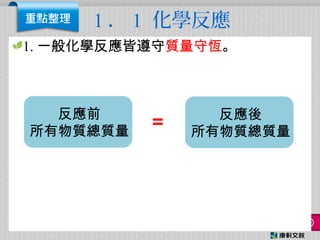
- 1. 1 . 1 化學反應 1. 一般化學反應皆遵守質量守恆。 反應前 所有物質總質量 反應後 所有物質總質量 =
- 2. 2. 碳酸鈉與氧化鈣反應,生成氯化鈉與碳酸鈣 沉澱,實驗前、後質量不變。 Na2CO3 + CaCl2→2NaCl + CaCO3 1 . 1 化學反應 白色 沈澱
- 3. 1 . 1 化學反應 3. 碳酸鈣與鹽酸反應,生成氯化鈣、水與二氧 化碳。 4. 若在未密閉的容器中反應,反應後質量會減 少,是因為生成的二氧化碳逸散到空氣中。 氣體 CaCO3 + 2HCl→CaCl2 + H2O + CO2
- 4. 1 . 1 化學反應 5. 鋼絲絨在空氣中燃燒後變重,是因為鐵與空 氣中的氧化合形成氧化鐵。 鐵 + 氧 → 氧 化鐵 重 量 增 加
- 5. 1 . 2 細數原子與分子 6. 國際上以質量數 12 的碳原子( C-12 )質量 作 為比較標準,定其原子量為 12 。 7. 分子中所含原子的原子量總和即為分子量。分子量 = 原子量總 和
- 6. 1 . 2 細數原子與分子 8. 莫耳︰表示物質粒子數的計量單位。 1 莫耳≒ 6×1023 個粒子 亞佛加厥數
- 7. 1 . 3 化學計量 9. 化學反應式的書寫流程︰ (1) 以適當的化學式表示反應物與生成物。 (2) 用箭號「→」表示反應進行方向,箭號左 邊是反應物,右邊是生成物。反應物與 生 成物不只一種時,以「+」連接。
- 8. 1 . 3 化學計量 (3) 平衡反應式:為使化學反應式能符合質量 守恆定律,必須在各化學式前加上係數 , 使箭號兩邊同種原子的數目相等。 (4) 若實驗在某種條件下進行,可在箭號上方 或下方加註說明,例如: 2H2O2 2H2O + O2 MnO2
- 9. 1 . 3 化學計量 10. 一個是否會反應必須根據實驗的結果決定 。 因此化學反應式是用來表達實驗結果,不 可 憑空杜撰。 11. 碳在空氣中完全燃燒會生成二氧化碳,化 學反應式表示如下:C + O2 → CO2
- 10. 1 . 3 化學計量 12. 化學反應式除了可以表示反應前、後的物 質種類,還可以表示參與反應的物質間, 粒子數量與質量的變化關係。
- 11. 1 . 3 化學計量 13. 反應式︰ 2H2 + O2 → 2H2O 平衡後氫、氧與水的反應式係數比 = 2 : 1 : 2 =分子數比 =莫耳數比 2 個氫分子 2 莫耳氫分子 1 個氧分子 1 莫耳氧分子 2 個水分子 2 莫耳水分子
















































